FIGURE 2.
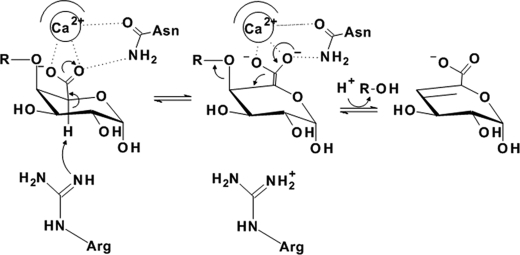
General β-elimination reaction mechanism for catalysis by pectate lyases. α1,4-Linked galacturonate substrates adopting the relaxed 4C1 chair conformation present the ideal geometry for an anti periplanar transelimination with the C5 hydrogen and C4 hydroxyl group both orientated in axial configurations. The rate-limiting step is the acidification and deprotonation of C5, a process that is facilitated by the effects of the electrophilic uronate group, catalytic divalent metals, and localized basic residues within the active site, which draw charge from the C5 carbon and increase the reactivity of its lone hydrogen atom. Abstraction occurs via general base attack by an arginine (family 1, 2, 3, and 10) or lysine (family 9) (43), the high pKa of these residues accounting for the basic pH optima observed across the PL landscape. Recently, the contributions of an ancillary lysine in family 1 PLs and asparagines in family 9 have been proposed to influence the catalytic rate by stabilizing the enolate-enolate intermediate (45). These residues have been implicated in hydrogen donation and/or bonding to the nascent oxyanion of the uronate group following electron transfer to the transient double bond between C5 and C6. Decomposition of the intermediate occurs by protonation of the scissile glycosidic oxygen and elimination of the axial O4 by electron shuttling from the C5–C6 bond to the C4–C5 bond of the sugar ring. This unsaturation distorts the relaxed chair structure of the pyranose because the trigonal planar geometry of C4 and C5 results in migration of C4 into the plane of C2, C3, and C5. The substrate is shown is a disaccharide, with the R-group depicting a second GalA or DKI molecule (note the Asn residue shown may be equivalent to Gln350).