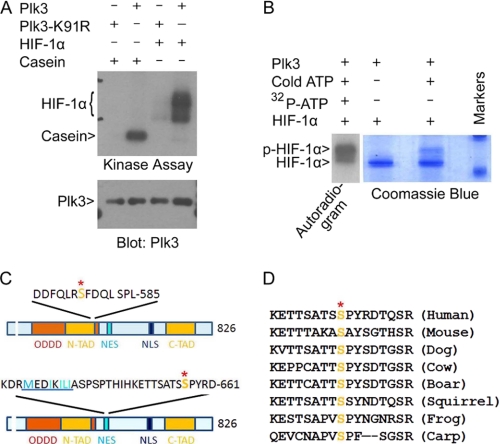
FIGURE 3.
Plk3 phosphorylates HIF-1α on Ser576 and Ser657. A, both Plk3 and Plk3-K91R were expressed in Sf9 cells and purified using NTA-Ni resin. Purified Plk3 and Plk3-K91R were assayed for their kinase activities toward HIF-1α in vitro in a kinase buffer containing [γ-32P]ATP. A representative autoradiogram is shown. B, kinase assays were carried out in the presence of various components as indicated. After the reaction, the samples were analyzed on SDS-PAGE followed by Coomassie Blue staining. A lane of “hot” sample (autoradiogram on the left) that ran on the same gel is also shown. C, HIF-1α sequence representation shows the relative positions of serine phosphorylation sites Ser576 (upper panel) and Ser657 (lower panel) to HIF-1α domains. ODDD, oxygen-dependent degradation domain; N-TAD, N-terminal transactivation domain; NES, nuclear export signal; NLS, nuclear localization signal; C-TAD, C-terminal transactivation domain. The nuclear export signal in the lower panel is highlighted by underlining. Ser641 and Ser643 are known to be phosphorylated by ERKs. D, amino acid alignment is shown of HIF-1α molecules from different species in the region that were targeted by Plk3. The conserved serine residues are highlighted.