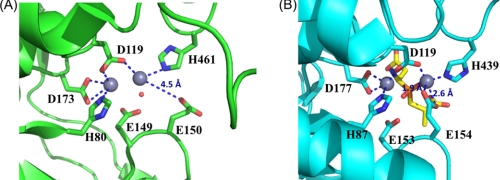
FIGURE 4.
Comparison of the active sites in V. alginolyticus PepD and structural homologs. A, local view of the di-zinc center of PepD. The residues involved in coordination of Zn1 and Zn2 (gray spheres) are shown as green sticks. B, local view of the di-zinc center of PepV. The residues involved in metal coordination are shown as cyan sticks, and the phosphinic inhibitor (AspΨ[PO2CH2]AlaOH) is represented by yellow sticks. In the PepD active site, Zn1 is coordinated by Asp119, His461, and a single putative water molecule hydrogen-bonded to the Glu149, whereas Zn2 is coordinated by His80, Asp119, and Asp173. The putative water molecule of PepD is depicted by a red dot. Asp119 of PepD serves as a bridging ligand for metal coordination.