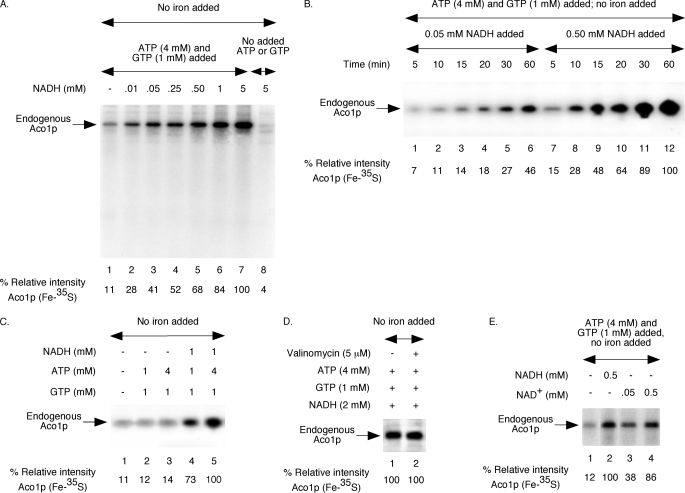
FIGURE 1.
NAD(H)-dependent Fe-35S labeling of endogenous aconitase in wild-type mitochondria. Each reaction mixture contained wild-type mitochondria (200 μg of proteins) and [35S]cysteine (10 μCi), and all assays were performed without added iron. A, assay mixtures were supplemented with ATP (4 mm) and GTP (1 mm) and/or different concentrations of NADH as indicated. Samples were incubated at 30 °C for 30 min and diluted with buffer A, and mitochondria were reisolated by centrifugation. Mitochondrial membranes were ruptured, and soluble proteins were analyzed by native PAGE followed by autoradiography (28, 29). The intensity of Aco1p (Fe-35S) in the presence of ATP (4 mm), GTP (1 mm), and NADH (5 mm) was arbitrarily considered 100% (lane 7). B, reaction mixtures were supplemented with ATP (4 mm), GTP (1 mm), and NADH (0.05 or 0.5 mm) as indicated. Samples were incubated at 30 °C for different time periods and analyzed as in A. C, NADH, ATP, and GTP were included as indicated. Samples were incubated at 30 °C for 30 min and analyzed as in A. D, mitochondria were pretreated with valinomycin for 5 min on ice as indicated. Following addition of ATP, GTP, and NADH, samples were incubated at 30 °C for 30 min and analyzed. E, reaction mixtures were supplemented with ATP (4 mm) and GTP (1 mm). NADH or NAD+ was included as indicated. Following incubation at 30 °C for 30 min, samples were analyzed.