Abstract
Vitamin D has been shown to have immunomodulatory function, but the molecular basis for it has not been well understood. In this study, we found that vitamin D receptor expression was induced in a CD4+ effector T cell lineage, Th17 cells, which required the transcription factors, RORα, RORγt, and STAT3. Treatment of mice with an active ligand of vitamin D receptor (VDR), 1,25-dihydroxyvitamin D3 (1,25D3), ameliorated experimental autoimmune encephalomyelitis, accompanied with reduced IL-17 and IL-17F expression. In vitro, treatment of CD4+ T cells with the physiological doses of 1,25D3 preferentially inhibited cytokine production by Th17 cells, in a VDR-dependent manner, without affecting the expression of transcription factors or surface molecules. Moreover, at these concentrations, cytokine expression was suppressed only at protein but not at mRNA levels. Stimulation of Th17 cells with 1,25D3, in a concentration-dependent manner, induced the expression of C/EBP homologous protein (CHOP), a molecule involved in endoplasmic reticulum stress and translational inhibition. In addition, overexpression of CHOP in developing Th17 cells suppressed their cytokine production. Our results suggest a novel, post-transcriptional mechanism whereby Th17 cytokines are inhibited by VDR, which may underscore future therapeutic usage of vitamin D in treatment of autoimmune diseases.
Keywords: Cell Differentiation, Cytokine, Inflammation, Protein Synthesis, Vitamin D
Introduction
Vitamin D is an essential nutrient that regulates calcium and phosphate transport and bone mineralization (1). In recent years, however, vitamin D has been found to have a much broader range of actions, including regulation of cell differentiation, proliferation, and apoptosis (2). The role of vitamin D in immune system is complex and diverse. The systemic or locally produced active form of vitamin D, 1,25-dihydroxyvitamin D3(1,25D3),3 can exercise its effects on several immune cells, including macrophages, dendritic cells, and T and B cells. Several reports indicated that 1,25D3 can act directly on T cells or indirectly on dendritic cells to modulate T cell function (3–6). However, the concentrations of 1,25D3 employed in many studies are often above the physiological range, bringing into question the physiological relevance of the effect of 1,25D3 on T cells. Nonetheless, modulation of CD4+ T cells by 1,25D3 is intriguing because several studies have demonstrated that 1,25D3 is capable of suppressing inflammation in vivo (7–10).
Recently, a new subset of CD4+ T cells, Th17 cells, was reported to have an essential role in dampening local inflammation (11–13). Th17 cells produce proinflammatory molecules, IL-17, IL-17F, and IL-22, which act on tissue resident cells to promote inflammation. TGFβ and IL-6 are critical in generation of Th17 cells by activating STAT3 and inducing the transcription factors, retinoic acid-related orphan receptor (RORγt) (14) and RORα (15). IL-1 and IL-23 are also important cytokines to stabilize and maintain Th17 cells (16, 17). Therefore, pathways to regulate Th17 cell development and its cytokines are relevant. Of interest, a previous report indicated that 1,25D3 is able to prevent experimental autoimmune uveitis, partially because of its suppressive effect directly on Th17 cells (10). However, it is not known whether the effect is mediated through vitamin D receptor (VDR) and how 1,25D3 suppresses IL-17 and other Th17 cytokine production in T cells.
In this study, we have examined the mechanism whereby 1,25D3 regulates CD4+ T cell function at physiological concentrations. We found that in response to 1,25D3, Th17 cells produced reduced levels of cytokines at the protein level, whereas their transcriptions were not affected. Moreover, 1,25D3 treatment in Th17 cells induced the expression of C/EBP homologous protein (CHOP). Overexpression of CHOP in Th17 cells suppressed their cytokine production. Our data thus suggest a post-transcriptional inhibition of Th17 cell function by VDR.
EXPERIMENTAL PROCEDURES
Mice
C57Bl/6- and VDR-deficient mice were purchased from The Jackson Laboratory, and 6–8-week-old mice were used in experiments. Mice were housed in the specific pathogen-free animal facility at MD Anderson Cancer Center, and the animal experiments were performed using protocols approved by the Institutional Animal Care and Use Committee.
Th Cell Differentiation
Naive CD4+CD25−CD62Lhi- CD44lo T cells from C57Bl/6 mice were FACS-sorted and stimulated with plate-bound anti-CD3 (0.5 μg/ml) plus soluble anti-CD28 (0.5 μg/ml) in the presence of 2 ng/ml TGFβ and 10 ng/ml IL-6. After 4 days of culture, cells were washed and restimulated with plate-bound anti-CD3 (1 μg/ml) for 4 h, and cells were then collected for RNA extraction. Different concentrations of 1,25D3 (Sigma) were added to culture at day 0. For cytokine measurement by ELISA, culture supernatants were collected for the analysis 24 h after anti-CD3 restimulation. For intracellular cytokine analysis, cells were restimulated with 500 ng/ml ionomycin and 50 ng/ml PMA in the presence of GolgiStop (BD Pharmingen) for 5 h. Cells were then permeabilized with Cytofix/Cytoperm kit (BD Pharmingen) and analyzed for the expression of IL-17 and IFNγ (BD Pharmingen).
EAE Induction
Mice were immunized subcutaneously at the dorsal flanks with 150 μg of Myelin oligodendrocyte glycoprotein (MOG) peptide emulsified in Complete Freund's adjuvant at days 0 and 7. Pertussis toxin was given intraperitoneally at days 1 and 8 (500 ng/injection). Signs of experimental autoimmune encephalomyelitis (EAE) were assigned scores on a scale of 1–5 as follows: 0, none; 1, limp tail or waddling gait with tail tonicity; 2, wobbly gait; 3, hind-limb paralysis; 4, hind-limb and forelimb paralysis; 5, death. Disease incidence and scores were measured daily. 50 ng of 1,25D3 was administered via an intraperitoneal route for every 3 days throughout EAE induction.
Quantitative Real-time RT-PCR
Total RNA was prepared from T cells with TRIzol reagent (Invitrogen). cDNA was synthesized with SuperScript reverse transcriptase and oligo(dT) primers (Invitrogen), and gene expression was examined with a Bio-Rad iCycler optical system with an iQ SYBR Green real-time PCR kit (Bio-Rad Laboratories). The data were normalized to Actb as a reference. The primers for Vdr are: forward, 5′-CTCCTCGATGCCCACCACAAGACCTACGT-3′, and reverse, 5′-GTGGGGCAGCATGGAGAGCGGAGACAG-3′. The primers for Il17, Il17f, Il22, Il23r, Rora, Rorc, and Actb were previously described (15).
Western Blot Analysis
Naive CD4+ T cells were cultured with TGFβ and IL-6 in the presence of 1,25D3. At day 5, cultured cells were collected and lysed in lysis buffer. Lysates were denatured with 2× SDS sample buffer before SDS-PAGE. Protein transfer was followed by overnight incubation with anti-CHOP (Santa Cruz Biotechnology) or anti-actin (Sigma). After incubation with HRP-conjugated secondary antibody, the signal was detected with ECL reagent (Promega).
Retroviral Transduction
The full length of cDNA encoding CHOP was cloned into pGFP-RV. Naive CD4+CD25−CD62LhiCD44lo T cells from C57Bl/6 mice were FACS-sorted and activated with plate-bound anti-CD3 and soluble anti-CD28 in the presence of polarizing cytokines. 36 h after activation, cells were spin-infected with retrovirus expressing pGFP or CHOP, and medium was replaced with Th17 conditioning medium after 4 h of infection. 4 days after activation, GFP+ cells were FACS-sorted, and gene expression was assessed by real-time RT-PCR. FACS-sorted cells were also restimulated with plate-bound anti-CD3 overnight, and the culture supernatants were analyzed by ELISA.
RESULTS AND DISCUSSION
Vdr Is Highly Expressed in Activated CD4+ T Cells
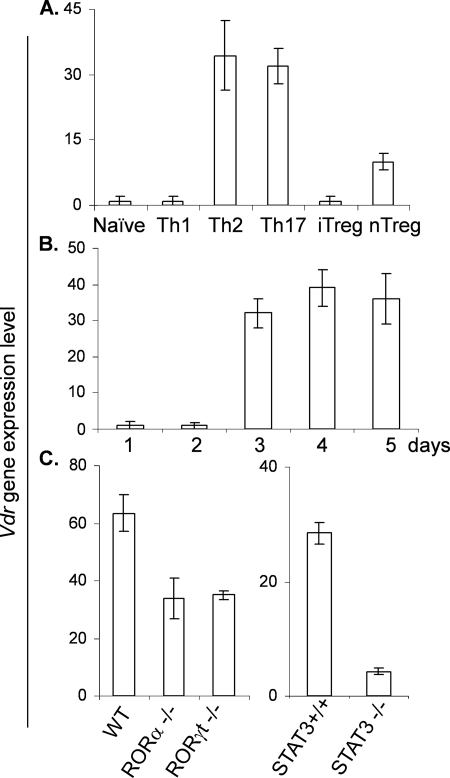
Because the active form of vitamin D, 1,25D3, has immunomodulatory effects on CD4+ T cells, including inhibition of Th17 cell function, we first determined whether Vdr mRNA is expressed in Th subsets. Sorted naive CD4+ T cells were cultured under different polarizing conditions, and Vdr expression level was examined by real-time RT-PCR. We found that although naive T cells or Th1 cells express a minimal level of Vdr, differentiated Th17 and Th2 cells, and to a less extent, Treg cells, expressed increased levels of Vdr mRNA (Fig. 1A). Induction of Vdr mRNA was observed at day 3 after culturing naive CD4+ T cells under Th17-inducing conditions in the presence of TGFβ and IL-6 (Fig. 1B). Expression of Vdr in Th17 cells requires the transcription factors that govern Th17 cell differentiation. RORα-, RORγt- and Stat3-deficient CD4+ T cells were partially defective in Vdr expression when they were polarized for Th17 cell differentiation (Fig. 1C).
FIGURE 1.
Differential expression of Vdr in the subsets of CD4+ T cells. A, mRNAs were isolated from different subsets of CD4+ T cells and subjected to real-time RT-PCR analysis. Relative mRNA expression was compared by setting the lowest expression amount to an arbitrary value of 1. iTreg, inducible Treg cells; nTreg, natural Treg cells. B, naive CD4+ T cells were cultured with TGFβ and IL-6. mRNAs were isolated at each time point and subjected to real-time RT-PCR. C, WT and RORα-, RORγt-, and STAT3-deficient mice were differentiated into Th17 cells, and mRNAs from these cells were subjected to real-time RT-PCR analysis. Data represent the means ± S.E. of at least three independent experiments.
1,25D3 Inhibits the Development of EAE
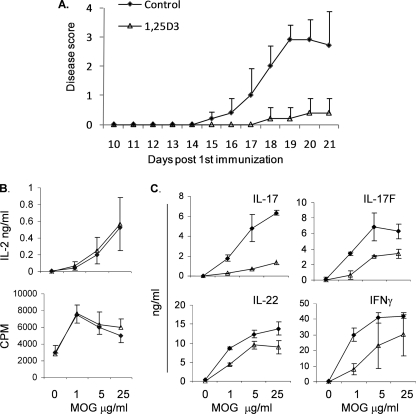
To better understand the function of VDR in autoimmunity, we treated mice with 50 ng of 1,25D3 for every 3 days during the development of EAE, an autoimmune disease model. The animals injected with 1,25D3 exhibited a reduction in disease symptoms and delayed disease incidence (Fig. 2A). When the splenocytes from non-treated and 1,25D3-treated animals were restimulated with MOG peptide, IL-2 production or proliferation of T cells remained similar between the control and treated groups. On the other hand, the production of IL-17 and IL-17F cytokines was severely impaired, whereas production of IL-22 and IFNγ was more moderately decreased in splenocytes from the 1,25D3-treated group than the control mice (Fig. 2B). These results indicate that 1,25D3 inhibits T cell effector cytokine expression but not their primary activation in vivo.
FIGURE 2.
1,25D3 treatment protected mice from development of EAE. A, clinical scores of control and 1,25D3-treated mice after EAE immunization. B, C, splenocytes from mice with EAE mice were collected and restimulated with MOG peptide for 3 days. The culture supernatants were analyzed by ELISA. Data represent the means ± S.E. of three independent experiments and at least four mice in each group.
1,25D3 Inhibits Th17 Cytokine Protein Production in vitro via VDR
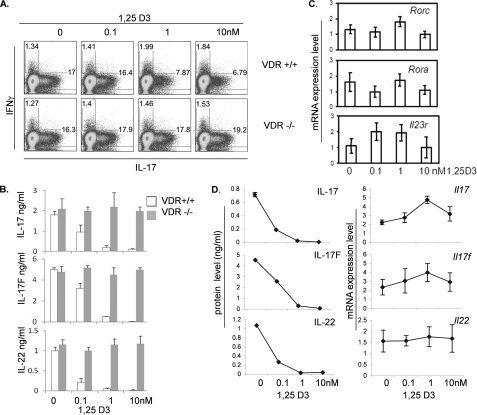
We next further examined the roles of 1,25D3 on effector T cell differentiation in vitro. In agreement with the previous studies (4, 5), treatment of 1,25D3 in the range of physiological concentrations did not have any effect on cytokine expression by Th1 cells, whereas IL-4 and IL-5 expression by Th2 cells was slightly increased within the 0.1–1 nm range of 1,25D3 (data not shown). On the other hand, 1,25D3 inhibited Th17 cytokine expression. Naive CD4+ T cells were cultured with TGFβ and IL-6, and the increasing concentrations of 1,25D3 were added to the cultures at day 0. Differentiated Th17 cells were restimulated at day 5 with PMA and ionomycin for intracellular cytokine staining or anti-CD3 for ELISA to determine the expression level of IL-17. A dose-dependent reduction in IL-17 production was observed by intracellular cytokine staining as well as ELISA (Fig. 3B). The expression of other Th17 cytokines IL-17F and IL-22 was also reduced in the same manner, as determined by ELISA. In contrast, when VDR-deficient naive CD4+ T cells were cultured in the Th17-polarizing condition with increasing concentrations of 1,25D3, VDR-deficient cells were resistant to 1,25D3 treatment, and the production of Th17 cytokines remained unchanged (Fig. 3A). This indicates that the actions of 1,25D3 in CD4+ T cells are VDR-dependent.
FIGURE 3.
Suppressive effect of 1,25D3 is VDR-dependent, and cytokine production is independent from transcriptional inhibition. A, flow cytometry plots of naive CD4+ T cells after culture in Th17-polarizing conditions for 5 days. Cells were activated with PMA/Ionomycin for 4 h and then stained for IL-17 and IFNγ. B, measurement of IL-17, IL-17F, and IL-22 in the supernatants of the cultures described in A. C, real-time RT-PCR analysis of the Rorc, Rora, and Il23r genes from T cells after culture under Th17-polarizing conditions. D, comparison of ELISA versus mRNA expression levels of Il17, Il17f, and Il22. Data represent the means ± S.E. of at least three independent experiments.
1,25D3 Does Not Inhibit Th17 Cytokine Gene Transcription
To better understand the mechanisms whereby 1,25D3 inhibits Th17 cytokine expression, we analyzed gene expression at the mRNA level. Naive CD4+ T cells were cultured in the presence of TGFβ and IL-6, and increasing concentrations of 1,25D3 were added into the cultures at day 0. Differentiated Th17 cells were restimulated with anti-CD3 at day 5 for 4 h, and mRNAs were collected for real-time RT-PCR analysis. mRNA expression level of Th17-related transcription factors Rorc and Rora or surface molecules such as Il23r remained the same at 0.1–10 nm of the ligand concentration (Fig. 3C). Levels of cytokine mRNA, Il17, Il17f, and Il22 were also similar at 0.1–10 nm of the ligand concentration (Fig. 3D). Although we observed reduction in mRNA expression levels of the cytokines and the transcription factors at higher concentrations of ligand (above 10 nm), 1,25D3 circulates in very low concentrations (about 25–275 pm) (18). Even with dietary vitamin supplement or massive UV exposure, they rarely reach 10 nm of concentration (19). Therefore, we considered 0.1–1 nm of the ligand concentrations physiologically relevant. In contrast to mRNA levels of the cytokines, when the cultured cells were restimulated with anti-CD3 for overnight and subjected to ELISA assay, reduction of the cytokines, IL-17, IL-17F, and IL-22, was observed at 0.1 nm of the ligand concentration (Fig. 3D). Moreover, ELISA assay of the cytokines showed a severe defect in cytokine production at 1 nm of the ligand concentration (Fig. 3D).
1,25D3-induced CHOP Suppresses Th17 Cytokine Production
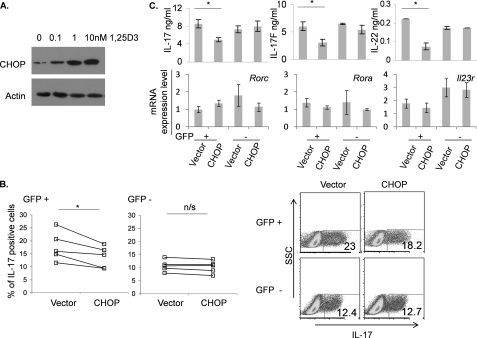
Reduction of the cytokines in protein expression level but not in mRNA expression level by 1,25D3 suggests the presence of the inhibitory mechanism of mRNA translation in CD4+ T cells. However, based on flow cytometry, expression of CD4 protein in the cells cultured with 1,25D3 was not affected (data not shown), suggesting that protein expression regulated by 1,25D3 is specific to cytokine molecules. Because CHOP/GADD153 (growth arrest and DNA damage-inducible protein 153) is known to inhibit translation and VDR has been shown to regulate CHOP transcription directly in several cell lines (20), we tested whether CHOP can be induced by 1,25D3 in CD4+ T cells. Naive CD4+ T cells were differentiated into Th17 cells in the presence of TGFβ and IL-6, and 1,25D3 was added to the cultures at day 0. After culturing the cells for 5 days, differentiated CD4+ T cells were collected, and cell lysates were analyzed for CHOP expression. Western blot analysis indicated that CHOP is induced in Th17 cells by 1,25D3 in a concentration-dependent manner (Fig. 4A). Induction of CHOP was specific to Th17 cells because 1,25D3 did not induce expression of CHOP in Th2 cells, although Th2 cells express higher levels of VDR in comparison with naive CD4 T cells (data not shown), suggesting that there are additional regulatory mechanisms underlying CHOP expression.
FIGURE 4.
CHOP is induced upon 1,25D3 treatments in Th17 cells, and overexpression of CHOP on Th17 cells leads to inhibition of cytokine production. A, naive CD4+ T cells were treated with increasing concentrations of 1,25D3 under Th17-inducing conditions. Cells were collected at day 5, lysates were analyzed by SDS-PAGE gel, and CHOP was detected using anti-CHOP antibody. B, naive CD4+ T cells were cultured under Th17-inducing conditions with or without retroviral overexpression of CHOP. Cells were stained at day 4 (*, p < 0.05). C, GFP-positive and -negative cells were sorted, and mRNAs were isolated for gene expression analysis by real-time RT-PCR. The sorted cells were cultured overnight with anti-CD3, and culture supernatants were subjected to ELISA. Data represent the means ± S.E. of at least three independent experiments. n/s, not significant.
To see whether CHOP can suppress Th17 cytokine production in CD4+ T cells, CD4+ T cells were overexpressed with CHOP using a retroviral vector. Naive CD4+ T cells were cultured with anti-CD3 and anti-CD28 for 2 days and transduced with CHOP or IRES-GFP for control. After transduction, CD4+ T cells were cultured under Th17-polarizing conditions with TGFβ and IL-6. At day 4, the cultured cells were stimulated with ionomycin and PMA to access expression level of IL-17. Intracellular cytokine staining showed that CHOP-overexpressing cells expressed a reduced level of IL-17 upon restimulation (Fig. 4B). To further analyze characteristics of CHOP-overexpressing Th17 cells, the cultured cells were sorted based on their GFP expression. After overnight culture with anti-CD3, culture supernatants were subjected to ELISA assay to access cytokine production. IL-17, IL-17F, and IL-22 were reduced in GFP-positive cells overexpressing CHOP as compared with the controls (Fig. 4C). To see whether the transcription factors in CHOP-overexpressing CD4+ T cells are regulated, mRNAs from the sorted GFP-positive and -negative cells were isolated to access gene expression level. mRNA levels of Rorc, Rora, and Il23r in CHOP transduced Th17 cells remained the same as the controls (Fig. 4C). CHOP-transduced Th17 cells did not exhibit significant apoptosis because binding to 7-aminoactinomycin D and annexin-V remained similar in these cells to the controls (data not shown). Although CHOP is involved in endoplasmic reticulum stress-induced apoptosis in some cell types (21), the role in T cells is not known. Nonetheless, induction of CHOP in T cells was reported previously and is considered as a part of integrated stress responses in T cells (22, 23). Together, these results indicate that induction of CHOP might be involved in suppression of Th17-related cytokine, which seems to be independent from the known Th17 differentiation program.
In summary, we have investigated the effect of the VDR pathway in Th17 cells. By inducing VDR during differentiation, TH17 cells become sensitive to being regulated by locally or systemically produced 1,25D3, the active form of vitamin D. At a lower concentration of 1,25D3, Th17 cells can suppress the production of cytokines without inhibiting their transcriptions. We propose that this may in part be due to induction of the endoplasmic reticulum stress protein, CHOP. Although it remains to be answered how CHOP mediates suppression of cytokines in Th17 cells, this report proposes a novel regulatory pathway of Th17 cytokines via vitamin D.
Acknowledgments
We thank the members of the Dong laboratory for help.
This work was supported, in whole or in part, by National Institutes of Health Grant 5R01AR050772-09 (to C. D.) and a Research Trust Fellowship (to C. D.).
- 1,25D3
- 1,25-dihydroxyvitamin D3
- VDR
- vitamin D receptor
- CHOP
- C/EBP homologous protein
- C/EBP
- CCAAT/enhancer-binding protein
- EAE
- experimental autoimmune encephalomyelitis
- PMA
- phorbol 12-myristate 13-acetate
- ROR
- retinoic acid-related orphan receptor
- MOG
- myelin oligodendrocyte glycoprotein.
REFERENCES
- 1.Holick M. F. (1996) J. Nutr. 126, 1159S–1164S [DOI] [PubMed] [Google Scholar]
- 2.Mora J. R., Iwata M., von Andrian U. H. (2008) Nat. Rev. Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery L. E., Burke F., Mura M., Zheng Y., Qureshi O. S., Hewison M., Walker L. S., Lammas D. A., Raza K., Sansom D. M. (2009) J. Immunol. 183, 5458–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staeva-Vieira T. P., Freedman L. P. (2002) J. Immunol. 168, 1181–1189 [DOI] [PubMed] [Google Scholar]
- 5.Mahon B. D., Wittke A., Weaver V., Cantorna M. T. (2003) J. Cell. Biochem. 89, 922–932 [DOI] [PubMed] [Google Scholar]
- 6.Sigmundsdottir H., Pan J., Debes G. F., Alt C., Habtezion A., Soler D., Butcher E. C. (2007) Nat. Immunol. 8, 285–293 [DOI] [PubMed] [Google Scholar]
- 7.Gorman S., Kuritzky L. A., Judge M. A., Dixon K. M., McGlade J. P., Mason R. S., Finlay-Jones J. J., Hart P. H. (2007) J. Immunol. 179, 6273–6283 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen L. B., Nashold F. E., Spach K. M., Hayes C. E. (2007) J. Neurosci. Res. 85, 2480–2490 [DOI] [PubMed] [Google Scholar]
- 9.Penna G., Amuchastegui S., Cossetti C., Aquilano F., Mariani R., Sanvito F., Doglioni C., Adorini L. (2006) J. Immunol. 177, 8504–8511 [DOI] [PubMed] [Google Scholar]
- 10.Tang J., Zhou R., Luger D., Zhu W., Silver P. B., Grajewski R. S., Su S. B., Chan C. C., Adorini L., Caspi R. R. (2009) J. Immunol. 182, 4624–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C. (2008) Nat. Rev. Immunol. 8, 337–348 [DOI] [PubMed] [Google Scholar]
- 12.Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007) Annu. Rev. Immunol. 25, 821–852 [DOI] [PubMed] [Google Scholar]
- 13.Zhou L., Littman D. R. (2009) Curr. Opin. Immunol. 21, 146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. (2006) Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 15.Yang X. O., Pappu B. P., Nurieva R., Akimzhanov A., Kang H. S., Chung Y., Ma L., Shah B., Panopoulos A. D., Schluns K. S., Watowich S. S., Tian Q., Jetten A. M., Dong C. (2008) Immunity 28, 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung Y., Chang S. H., Martinez G. J., Yang X. O., Nurieva R., Kang H. S., Ma L., Watowich S. S., Jetten A. M., Tian Q., Dong C. (2009) Immunity 30, 576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langrish C. L., Chen Y., Blumenschein W. M., Mattson J., Basham B., Sedgwick J. D., McClanahan T., Kastelein R. A., Cua D. J. (2005) J. Exp. Med. 201, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platz E. A., Hankinson S. E., Hollis B. W., Colditz G. A., Hunter D. J., Speizer F. E., Giovannucci E. (2000) Cancer Epidemiol. Biomarkers Prev. 9, 1059–1065 [PubMed] [Google Scholar]
- 19.Vieth R. (1999) Am. J. Clin. Nutr. 69, 842–856 [DOI] [PubMed] [Google Scholar]
- 20.Tavera-Mendoza L., Wang T. T., Lallemant B., Zhang R., Nagai Y., Bourdeau V., Ramirez-Calderon M., Desbarats J., Mader S., White J. H. (2006) EMBO Rep. 7, 180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyadomari S., Mori M. (2004) Cell Death Differ. 11, 381–389 [DOI] [PubMed] [Google Scholar]
- 22.Sundrud M. S., Koralov S. B., Feuerer M., Calado D. P., Kozhaya A. E., Rhule-Smith A., Lefebvre R. E., Unutmaz D., Mazitschek R., Waldner H., Whitman M., Keller T., Rao A. (2009) Science 324, 1334–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn D. H., Sharma M. D., Baban B., Harding H. P., Zhang Y., Ron D., Mellor A. L. (2005) Immunity 22, 633–642 [DOI] [PubMed] [Google Scholar]