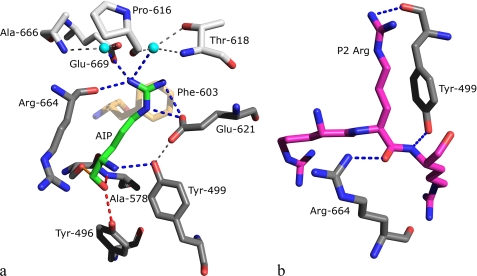
FIGURE 6.
The S1 (a) and S2 (b) binding pockets of OPB. Amino acids are shown in stick mode colored according to atom type: (N, blue and O, red), the color of the carbon atoms differs depending on the residue type. a, residues found directly binding to the P1 residue are colored gray, those indirectly binding are colored silver, Phe-603 is colored sand, and the P1 Arg is colored green. Waters are depicted as cyan spheres. Hydrogen bonds in the oxyanion binding site are represented by red dashed lines and covalent adduct is shown as solid orange line. Direct hydrogen bonds to the P1 Arg are represented by blue dashed lines, and indirect bonds in the binding pocket are shown as thin gray dashed lines. For clarity, only the P1 residue of antipain is shown, and Trp-625 is omitted from the figure. Glu-621 is the only residue found to hydrogen bond to the P1 guanidino group via its side chain. b, modeled tri-Arg peptide in the S2 binding pocket of OPB. Residues and the inhibitor are colored with gray or magenta C atoms, respectively. Blue dashed lines represent hydrogen bonds. The modeled P2 Arg was found to form a stacking interaction with Try-499; no other interactions were found using alternate rotamers.