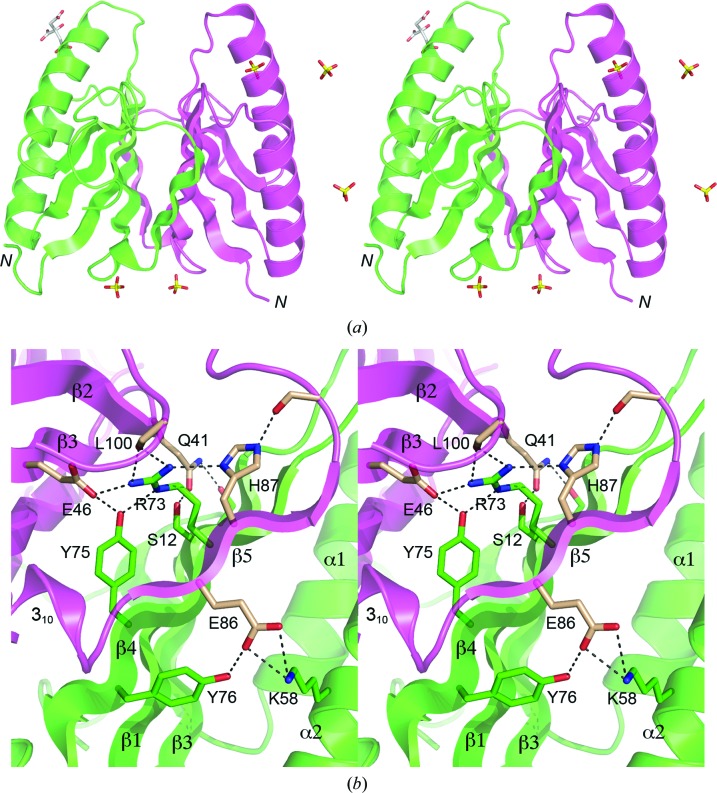
Figure 2.
Stereoviews of the DvuCas2 dimer interface. (a) The folds of the A and B protomers are rendered in magenta and green, respectively. The N-termini are indicated. Five sulfates and a citrate located at various sites on the protein surface are shown as stick models. The two sulfate sites at the bottom of the image were refined as partially occupied by sulfate and partially by citrate; only the sulfates are shown for the sake of clarity. (b) This view highlights the hydrophilic cross-protomer side-chain interactions. The secondary-structure elements are labeled as in Fig. 1 ▶(f). Side chains of the A and B protomers that comprise the dimer interface are shown as stick models with beige and green C atoms, respectively. Ionic and hydrogen-bonding contacts of side-chain atoms at the dimer interface are indicated by dashed lines.