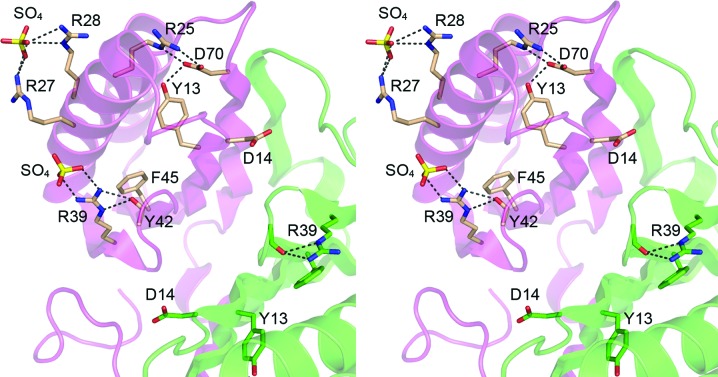
Figure 3.
Disposition of DvuCas2 residues corresponding to those implicated in the endoribonuclease activity of Cas2 homologs. The folds of the A and B protomers are rendered in magenta and green, respectively. The side chains of the A and B protomers that correspond to the putative Sso1404 phosphodiesterase active-site residues (Fig. 1 ▶ f) are shown as stick models with beige and green C atoms, respectively. Two sulfate anions docked on the surface of the DvuCas2 A protomer are also shown as stick models. Ionic and hydrogen-bonding interactions are indicated by dashed lines.