A recombinant multiple cofactor-dependent DNA ligase from S. zilligii has been purified and crystallized. X-ray diffraction data were collected to 2.9 Å resolution and the crystals belonged to space group P1.
Keywords: multiple cofactors, DNA ligases, Sulfophobococcus zilligii
Abstract
A recombinant DNA ligase from Sulfophobococcus zilligii that shows multiple cofactor specificity (ATP, ADP and GTP) was expressed in Escherichia coli and purified under reducing conditions. Crystals were obtained by the microbatch crystallization method at 295 K in a drop containing 1 µl protein solution (10 mg ml−1) and an equal volume of mother liquor [0.1 M HEPES pH 7.5, 10%(w/v) polyethylene glycol 10 000]. A data set was collected to 2.9 Å resolution using synchrotron radiation. The crystals belonged to space group P1, with unit-cell parameters a = 63.7, b = 77.1, c = 77.8 Å, α = 83.4, β = 82.4, γ = 74.6°. Assuming the presence of two molecules in the unit cell, the solvent content was estimated to be about 53.4%.
1. Introduction
DNA ligases catalyze the formation of a phosphodiester bond between 5′-phosphoryl and 3′-hydroxyl termini at single-strand breaks in double-stranded DNA. Sealing of single-strand breaks in duplex DNA is critical for maintaining genomic integrity during DNA-excision repair (Wood et al., 1988 ▶), DNA replication (Li & Kelly, 1984 ▶) and DNA recombination (Jessberger & Berg, 1991 ▶). DNA ligation proceeds through a highly conserved three-step reaction (Lehman, 1974 ▶). In the first step, the DNA ligase attacks the α-phosphorus of a cofactor, such as ATP or NAD+, releasing pyrophosphate or nicotinamide mononucleotide and forming a covalent ligase-adenylate intermediate in which AMP is bound to the ∊-amino group of a lysine through a phosphoamide bond. In the second step, the adenylyl group is transferred from the enzyme to DNA in order to generate a new pyrophosphate linkage between AMP and the 5′-phosphoryl terminus and form DNA-adenylate. Finally, the 5′-phosphate end of the nucleic acid strand is attacked by the apposing 3′-OH group to form a phosphodiester bond and release AMP.
On the basis of the cofactor necessary for the formation of the ligase-adenylate intermediate, DNA ligases have been grouped into two families: ATP-dependent and NAD+-dependent DNA ligases (Timson & Wigley, 1999 ▶; Sriskanda et al., 2000 ▶). Archaeal DNA ligases were classified as ATP-dependent DNA ligases. However, this classification has recently been challenged by archaeal DNA ligases that use multiple nucleotide cofactors. DNA ligases from Thermococcus fumicolans (Rolland et al., 2004 ▶), T. kodakaraensis KOD1 (Nakatani et al., 2000 ▶), T. onnurineus NA1 (Kim et al., 2006 ▶) and Pyrococcus abyssi (Rolland et al., 2004 ▶) use ATP and NAD+ and a DNA ligase from Aeropyrum pernix uses ATP and ADP (Jeon & Ishikawa, 2003 ▶). Furthermore, a recent study revealed that a DNA ligase from Sulfophobococcus zilligii (Szi DNA ligase; Sun et al., 2008 ▶) can use ATP, ADP and GTP as cofactors.
Ancestral DNA ligases are thought to use ADP, a low free-energy source, as a cofactor and structural features that can interact with higher energy sources such as ATP or NAD+ seem to have been acquired during the evolutionary process (Keppetipola & Shuman, 2005 ▶). From this viewpoint, the cofactor promiscuity of the Szi DNA ligase raises an interesting question concerning the evolution of DNA ligases in terms of cofactor utilization (Sun et al., 2008 ▶). Sequence comparison among archaeal DNA ligases does not reveal any specific motif that can explain how the Szi DNA ligase exhibits multiple cofactor specificity (Sun et al., 2008 ▶). Thus, the three-dimensional structure will provide invaluable insights into the molecular basis of the cofactor promiscuity of the Szi DNA ligase. Here, we report the purification, crystallization and preliminary crystallographic analysis of the Szi DNA ligase as a first step towards structure determination.
2. Materials and methods
2.1. Expression and purification of Szi DNA ligase
The Szi DNA ligase was cloned into pET-22b(+) expression vector (Novagen, Madison, Wisconsin, USA) to produce recombinant protein with a hexahistidine tag at the C-terminus. The plasmid was transformed into Escherichia coli BL21-Codon Plus (DE3)-RIL cells (Stratagene, La Jolla, California, USA) for protein expression. The transformed cells were grown in Luria–Bertani medium (Merck) containing 50 µg ml−1 chloramphenicol and 100 µg ml−1 ampicillin to an OD600 of 0.6 at 310 K and expression of the Szi DNA ligase was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (Duchefa). After 6 h induction at 310 K, the cells were harvested by centrifugation (5000 rev min−1, 10 min, 277 K). The harvested cells were washed with lysis buffer (20 mM Tris–HCl pH 7.5, 500 mM NaCl) and stored at 203 K until use. The frozen cells were resuspended in lysis buffer. Subsequently, the cells were disrupted by sonication and the crude lysate was centrifuged at 20 000g for 60 min at 277 K. The resulting supernatant was heat-treated at 353 K for 30 min and centrifuged in the same way as before. The clear supernatant was loaded onto an Econo-Column chromatography column (Bio-Rad) packed with 20 ml nickel–nitrilotriacetic acid (Ni–NTA) resin (Qiagen). The column was washed with two column volumes of washing buffer consisting of 20 mM Tris–HCl pH 7.5, 500 mM NaCl and 30 mM imidazole. The Szi DNA ligase was eluted with elution buffer consisting of 20 mM Tris–HCl pH 7.5, 500 mM NaCl and 500 mM imidazole. The ∼20 ml eluted fraction containing the Szi DNA ligase was concentrated to 5 ml and loaded onto a Superdex 75 HR 16/60 column (Amersham Biosciences) pre-equilibrated with a buffer consisting of 20 mM Tris–HCl pH 7.5, 150 mM NaCl and 2.5 mM dithiothreitol. The Szi DNA ligase eluted at ∼41 min at a flow rate of 1.5 ml min−1. The fractions containing the protein were pooled and concentrated to 20 mg ml−1 for crystallization screening. The protein purity was assessed to be >90% by scanning densitometry of Coomassie Blue-stained protein on a 12% sodium dodecyl sulfate polyacrylamide gel.
2.2. Microbatch crystallization and X-ray data collection
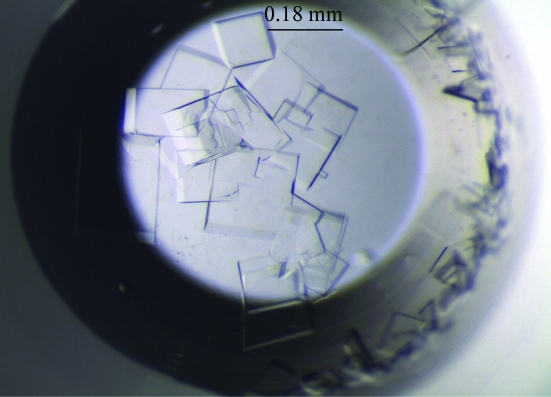
Crystallization screening was performed with commercially available screening kits from Hampton Research, Emerald BioStructures and Axygen Biosciences using the microbatch crystallization method. Firstly, crystallization reagents (1 µl) were pipetted into the wells of 96-well IMPACT plates (Greiner Bio-One) using a CyBi-Well pipettor (CyBio). Secondly, a layer of a 1:1 mixture of silicon oil and paraffin oil (5 ml) was poured onto the plate. Finally, protein solutions (1 µl) were manually pipetted under the oil layer. Initial crystals were grown in a precipitant consisting of 0.1 M HEPES pH 7.5 and 20%(w/v) polyethylene glycol (PEG) 10 000 (condition No. H2 of Crystal Screen 2 from Hampton Research). Further manual screens to determine the optimal crystallization conditions were performed using the microbatch crystallization method in 72-well HLA plates (Nunc), varying the buffer pH, the precipitant concentration and the protein concentration. Crystals of dimensions 0.18 × 0.16 × 0.05 mm (Fig. 1 ▶) that were suitable for diffraction experiments were obtained at 295 K in drops consisting of 1 µl protein solution (10 mg ml−1) and 1 µl mother liquor [0.1 M HEPES pH 7.5 and 10%(w/v) PEG 10 000; Fig. 1 ▶]. In an attempt to prepare crystals of the Szi DNA ligase complexed with ADP, ATP or GTP, cocrystallization was attempted using the same crystallization conditions. However, no crystals grew, indicating that nucleotide binding may induce substantial conformational change. As an alternative, we are attempting to determine new crystallization conditions for the ligase–nucleotide complexes.
Figure 1.
Crystals of the Szi DNA ligase.
A crystal was mounted using a nylon loop (10 µm Mounted Cryoloop from Hampton Research) for data collection and was frozen at 100 K using a Cryostream cooler (Oxford Cryosystems) after brief soaking in cryoprotectant solution consisting of 20% ethylene glycol, 0.1 M HEPES pH 7.5, 10% PEG 10 000. A 2.9 Å resolution data set was collected using an ADSC Quantum 315 CCD on beamline 4A of Pohang Light Source, Republic of Korea (Table 1 ▶). The exposure time to the synchrotron radiation was 5 s. A total of 360 frames of 1° oscillation were measured with the crystal-to-detector distance set to 350 mm. Diffraction data were processed using DENZO and scaled using SCALEPACK from the HKL-2000 program suite (Otwinowski & Minor, 1997 ▶). The data statistics are shown in Table 1 ▶.
Table 1. Data statistics for the Szi DNA ligase.
Values in parentheses are for the highest resolution shell.
| Space group | P1 |
| Unit-cell parameters (Å, °) | a = 63.7, b = 77.1, c = 77.8, α = 83.4, β = 82.2, γ = 74.6 |
| Wavelength (Å) | 0.99 |
| Resolution (Å) | 50–2.9 (3.0–2.9) |
| Completeness (>0σ) (%) | 92.8 (81.0) |
| Rmerge† (%) | 6.1 (13.2) |
| Average I/σ(I) | 15.3 (9.9) |
| Unique reflections | 29444 (2544) |
| Average multiplicity | 3.0 (2.2) |
| Mosaicity (°) | 0.9 |
R
merge = 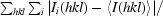
 , where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
, where I
i(hkl) is the ith intensity measurement of reflection hkl, including symmetry-related reflections, and 〈I(hkl)〉 is its average.
3. Results
The crystals of the Szi DNA ligase belonged to the triclinic space group P1, with unit-cell parameters a = 63.7, b = 77.1, c = 77.8 Å, α = 83.4, β = 82.4, γ = 74.6° (Table 1 ▶). Specific volume calculations (Matthews, 1968 ▶) based on the unit-cell parameters and molecular weight suggest that there could be two molecules in the unit cell, with a V M value of 2.7 Å3 Da−1 and a solvent content of 53.4%. Phasing information was obtained by molecular replacement (MR) using the program MOLREP. The structure of the ATP-dependent DNA ligase from Sulfolobus solfataricus (PDB code 2hiv; Pascal et al., 2006 ▶), which exhibits ∼61% sequence identity to the Szi DNA ligase, was used as a search model. The S. solfataricus ligase is composed of three domains: the adenylation domain (AdD), the N-terminal DNA-binding domain (DBD) and the C-terminal OB-fold domain (OBD). For MR, the three domains were used separately. The two positions of the DBD were first determined and were then fixed in the subsequent search for the positions of the AdD. After fixing the identified positions of the DBD and AdD, the two positions of the OBD were determined. Finally, we were able to obtain promising positions of two ligase molecules with good packing interactions in the asymmetric unit. Refinement and manual refitting of the initial model are now in progress.
Acknowledgments
This work was supported by the KORDI in-house programme (PE98513), the Marine and Extreme Genome Research Center programme and the Development of Biohydrogen Production Technology Using Hyperthermophilic Archaea programme of the Ministry of Land, Transport and Maritime Affairs, Republic of Korea.
References
- Jeon, S.-J. & Ishikawa, K. (2003). FEBS Lett.550, 69–73. [DOI] [PubMed]
- Jessberger, R. & Berg, P. (1991). Mol. Cell. Biol.11, 445–457. [DOI] [PMC free article] [PubMed]
- Keppetipola, N. & Shuman, S. (2005). J. Bacteriol.187, 6902–6908. [DOI] [PMC free article] [PubMed]
- Kim, Y. J., Lee, H. S., Bae, S. S., Jeon, J. H., Yang, S. H., Lim, J. K., Kang, S. G., Kwon, S.-T. & Lee, J.-H. (2006). Biotechnol. Lett.28, 401–407. [DOI] [PubMed]
- Lehman, I. R. (1974). Science, 186, 790–797. [DOI] [PubMed]
- Li, J. J. & Kelly, T. J. (1984). Proc. Natl Acad. Sci. USA, 81, 6973–6977. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Nakatani, M., Ezaki, S., Atomi, H. & Imanaka, T. (2000). J. Bacteriol.182, 6424–6433. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Pascal, J. M., Tsodikov, O. V., Hura, G. L., Song, W., Cotner, E. A., Classen, S., Tomkinson, A. E., Tainer, J. A. & Ellenberger, T. (2006). Mol. Cell, 24, 279–291. [DOI] [PubMed]
- Rolland, J.-L., Gueguen, Y., Persillon, C., Masson, J.-M. & Dietrich, J. (2004). FEMS Microbiol. Lett.236, 267–273. [DOI] [PubMed]
- Sriskanda, V., Kelman, Z., Hurwitz, J. & Shuman, S. (2000). Nucleic Acids Res.28, 2221–2228. [DOI] [PMC free article] [PubMed]
- Sun, Y., Seo, M. S., Kim, J. H., Kim, Y. J., Kim, G. A., Lee, J. I., Lee, J.-H. & Kwon, S.-T. (2008). Environ. Microbiol.10, 3212–3224. [DOI] [PubMed]
- Timson, D. J. & Wigley, D. B. (1999). J. Mol. Biol.285, 73–83. [DOI] [PubMed]
- Wood, R. D., Robins, P. & Lindahl, T. (1988). Cell, 53, 97–106. [DOI] [PubMed]