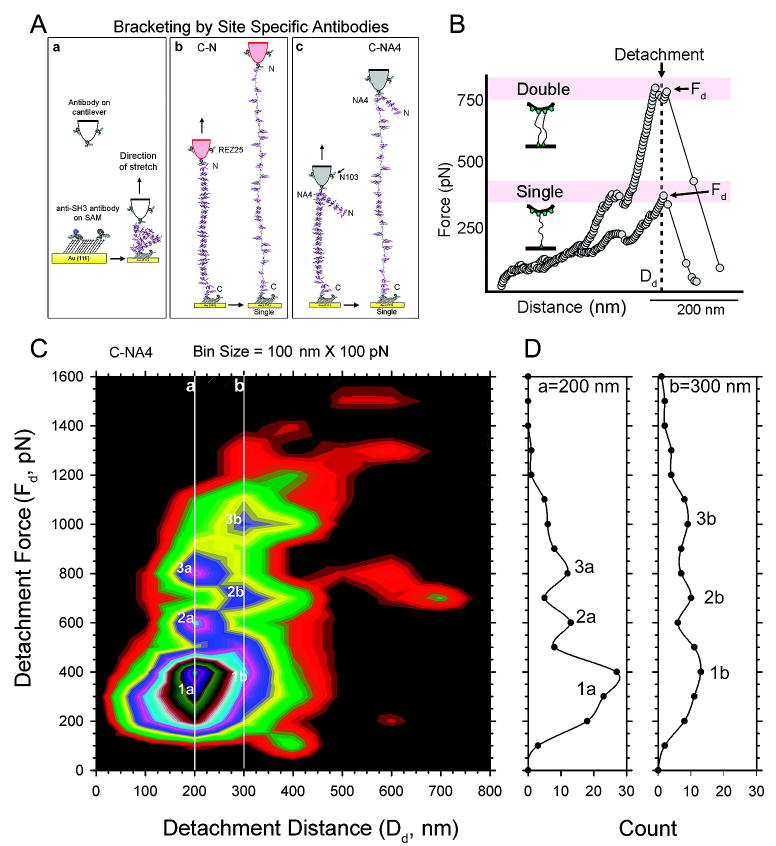
Figure 3.
Stretching nebulin molecules between site-specific antibodies on a SAM. (A) Stretching single nebulin molecules tethered between two pairs of site-specific antibodies. Anti-SH3 antibodies (REZ16) are attached sparsely to a functionalized OEG-SAM on an ultraflat gold surface. Antibodies to either the N-terminus (REZ25) or the NA4 epitope (N103) are conjugated to a functionalized AFM cantilever (a). These antibody pairs thus tether and stretch nebulin molecules and measure the elasticity of two segments C-N (b) or C-NA4 (c) of the same protein. The nonbracketed region (N-NA4 in the C-NA4 bracket) is not stretched. (B) Representative mountain range force spectra of antibody tethered nebulin. The molecule detaches (arrows) when the detachment force, Fd, reaches the lowest unbinding force of the antibodies (300–400 pN). Stretching two molecules in parallel gives rise to a proportionally higher detachment force Fd. (C) Two-dimensional histogram of 500 detachment points. Detachment forces (Fd) with a bin size of 100 pN vs detachment distance (Dd) with a bin size of 100 nm. Note the peaks in the histogram are related to each other by the proportionally higher force values for both the 200 nm (line a) and 300 nm (line b) detachment distance. (D) Traces through Dd = 200 nm (line a) and Dd = 300 nm (line b) showing multiple peaks of Fd for these two Dd values.