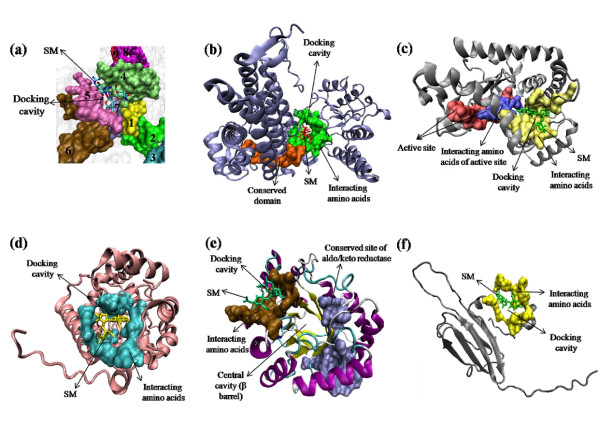
Figure 3.
3D models of overexpressed proteins showing docking with SM. Residues constituting interacting site, active site and conserved site are represented as space filled models with rest of the structure represented by cartoon structures. (a) Rv0350: Nine motifs are marked by numerals, docking cavity and SM are indicated by arrows and motifs 4, 5 & 7 are interacting with SM. (b) Rv0440: Red coloured SM, green coloured cavity and orange coloured conserved domain has been marked. Conserved domain is in the close vicinity of interacting site. (c) Rv1240: SM (green) is interacting with active site residues (blue) and other residues in the close vicinity (yellow) in the cavity. (d) Rv3075c: SM (yellow) interacting clearly with the central cavity residues (blue) of the globular protein. (e) Rv2971: SM (green) binding at the opposite side (brown) from conserved site of aldo/keto reductase (light blue) in the protein. Central cavity is present in the middle of complete β-barrel. (f) Rv2031c: SM (green) interacting with the outer part (yellow) of the protein in place of conserved HSP20-like chaperone domain.