Abstract
There is growing concern over the increasing use of opioids to treat chronic pain in the elderly primarily because of the potential increased sensitivity to the adverse side effects. Here, we use a preclinical model (male Brown Norway X F344 rats aged 12, 18, 24, and 30 months) to describe the outcome of chronic fentanyl administration (1.0 mg/kg/day) on various physiological and behavioral measures. Continuous fentanyl administration resulted in an initial decrease in food consumption, followed by the development of tolerance to this effect over a 4-week period and a subsequent increase in food consumption during withdrawal. This change in food consumption was associated with decreases in body weight (predominantly due to a loss of fat mass) that was maintained through early withdrawal. After one month of withdrawal, only the 12-month old animals had fully regained body weight. Fentanyl administration resulted in a decrease in grip strength and an increase in locomotor activity that did not differ across age groups. There was no effect of fentanyl administration on rotarod performance. These results demonstrate that while there is a delayed recovery of body mass with age, the observed changes in behavioral responses are uniform across ages.
Keywords: Locomotor activity, Rotarod, Grip strength, Body composition, Osmotic minipump
Introduction
Opioids have been used to treat essentially every type of pain in humans, including acute and chronic pain, for pre-, peri-, and post-surgical situations, for both cancer and non-malignant conditions, and their use is increasing and becoming more widely accepted (Atluri et al., 2003; Bell et al., 2009; Benyamin et al., 2008; Brixner et al. 2006; Garcia del Pozo et al., 2008; Pergolizzi et al., 2008; Trescot et al., 2008). This is especially relevant to the aged population where chronic pain due to diffuse conditions (Delgado-Guay & Bruera, 2008; Fine, 2001, 2004; Pergolizzi et al., 2008) such as neuromuscular pain (Helme & Gibson, 2001; Thomas et al., 2004) and arthritis (Donald & Foy, 2004), and to disease-related conditions such as cancer (Potter & Higginson, 2004; Rao & Cohen, 2004) is more prevalent, with estimates of as many as 40% of aged individuals in the community (e.g. > 50 years of age) and 80% in nursing homes (mean age of 75 years) reporting pain that interferes with daily functioning (Fox et al., 1999; Gagliese, 2009; Rustøen et al., 2005; Scudds and Robertson, 2000; Thomas et al., 2004; Zarit et al., 2004). Unfortunately, this pain is often undertreated in aged individuals (Auret & Schug, 2005; Bernabei et al., 1998; Chodosh et al., 2004; Gianni et al., 2009; McNeill et al., 2004). This problem is due in part to the fact that physicians are often reluctant to prescribe opioids to the elderly, given that the full spectrum of adverse side effects (e.g. nausea, constipation, and sedation)(Benyamin et al., 2008; Byas-Smith et al., 2005; Herndon et al., 2002; Swegle & Logemann, 2006) in this particular population is not known (Hutchinson et al. 2007; Lin et al., 2007; Thomason et al., 1998; Wilder-Smith, 2005).
Long-term studies of aging in the human population can be difficult and costly. However, preclinical studies have demonstrated that various behavioral and physiological measures in animals can function as correlates to human measures of declining physical function (Carter et al., 2002; Moser, 2000). These tests evaluate not only basic health metrics such as body weight and composition (fat mass versus muscle mass), but also physical performance measures related to muscle strength, agility, and overall activity. The purpose of the present study was to apply these evaluation tools to study the effects of chronic opioid administration and withdrawal on physical function in rats of various ages. While there are numerous opioids that are used for the treatment of pain, fentanyl is increasingly used for a wide range of conditions including epidural anesthesia, chronic back pain, and cancer pain (Bhambhani et al., 2010; Bell et al., 2009; de Leon-Casasola, 2008; Hong et al., 2010; Manchikanti & Singh, 2008; Pergolizzi et al., 2008; Rauck et al., 2009), and the development of novel formulations and delivery systems such as the transdermal patch makes fentanyl easy to administer chronically in outpatient situations (Grape et al., 2010). In the current study, fentanyl was continuously administered at a dose of 1.0 mg/kg/day for 28 days via osmotic minipumps, as this dosing protocol has previously been shown to produce antinociception (i.e. pain relief) in rats across a wide age range (Morgan et al., 2008; unpublished data). Increased understanding of how age influences the effects of opioids can result in minimizing adverse outcomes, and consequently lead to more effective pain management in the elderly.
Materials and methods
Animals, treatment conditions, and experimental design
Male Fisher 344 x Brown Norway rats, obtained from the National Institute of Aging colony at Harlan Industries (Indianapolis, IN) across four age groups (12, 18, 24, 30 months of age during baseline testing) were used in the present study. This range of ages represents adulthood, middle-age, pre-senescent and senescent portions of the lifespan. Animals were individually-housed in a temperature- and humidity-controlled colony room with a 12-hr light/dark cycle (lights on at 6 AM) with food and water available ad libitum. All surgery and testing was performed during the light cycle. Animals were cared for in accordance with the regulations of the IACUC and with the “Guide for the Care and Use of Laboratory Animals” (ILAR, 1996). In addition, animals were assessed on a weekly basis for signs of overt health problems with measures including, but not limited to, sudden decline in body weight, redness around the eyes and nostrils, ruffled coat, open sores on the tail, and haunched posture.
The experimental timeline is shown in the Table. In brief, upon arrival in the colony, the animals were given 2 weeks to acclimate before baseline testing began for body composition, grip strength, and open field with 2-3 days between each test. Animals within each age group were then randomized to receive osmotic mini-pumps containing either fentanyl (n = 32) or saline (n = 36). After four weeks of drug administration pumps were removed. In the end, 27 fentanyl- and 35 saline-treated animals completed the entire study (12 month: 5 fentanyl, 9 saline; 18 month: 9 fentanyl, 9 saline; 24 month: 7 fentanyl, 9 saline; 30 month 6 fentanyl, 8 saline). Six animals did not complete the experiment presumably due to an adverse interactions between fentanyl and isoflurane. Of primary interest were the behavioral and physiological effects of fentanyl at approximately one week and one month of chronic fentanyl administration. These time points were chosen to be analogous to either short-term treatment regimens related to surgery or outpatient clinical situations, or longer-term disease conditions associated with chronic pain. For ease of description, these time points are referred to as “early” and “late” periods of chronic drug administration or withdrawal. Behavioral tests were conducted on different days, and the order of animal testing was counterbalanced across ages. In general, the duration of the testing was less than 3 hours, and was conducted in the middle of the light/inactive phase.
Table 1.
Timelime of experimental events
| Phase | Day | Experimental event |
|---|---|---|
| Baseline | 0 | Arrival in lab |
| 14-42 | Open field, grip strength, TD-NMR, Rotorod, food consumption, and body weight measures | |
| | ||
| Drug / saline administration | 0 | Implant pump |
| 7 | Body weight, food consumption, Rotorod | |
| 8 | TD-NMR | |
| 10 | Open field, grip strength, TD-NMR, Rotorod, food consumption, and body weight measures | |
| 11 | Grip strength | |
| | ||
| 21 | Rotarod | |
| 22 | TD-NMR | |
| 24 | Open field | |
| 25 | Grip strength | |
| | ||
| 28 | Food consumption, body weight | |
| Withdrawal | 0 | Remove pump |
| 6 | Body weight | |
| 7 | Food consumption, TD-NMR | |
| | ||
| 21 | Rotarod | |
| 22 | TD-NMR | |
| 24 | Open field | |
| 25 | Grip strength | |
| 28 | Food consumption, body weight | |
Surgery and Drug Delivery
Osmotic mini-pumps (Model # 2ML4, Alzet, Durect Corp., Cupertino, CA) containing fentanyl or saline were implanted subcutaneously in the right hindquarter of animals while maintained on isoflurane anesthesia (1.5% at 1.0 L/min O2) . Mini-pumps delivered fluid at a rate of 2.58 μl/hr for 28 days. Fentanyl was delivered at a dose of 1 mg/kg/day. Four weeks after pump implantation, animals were anesthetized and the pumps removed.
Behavioral and Physiological Testing
Food Consumption
Twenty-four hour food consumption data were collected at 7 and 28 days after pump-implantation.
Body Weight and Composition
Body weight was measured weekly for all animals. Determination of body composition was assessed by time domain-nuclear magnetic resonance (TD-NMR) using a Minispec analyzer (Bruker Optics, The Woodlands, TX). TD-NMR testing allows for rapid (approximately 1 min) assessment of body composition in awake, restrained animals. Absolute values for fat, lean, and fluid mass were recorded. At each time point, each animal was tested twice and the average of those results is reported.
Open-field activity
Animals were placed into Plexiglas open-field testing chambers (690 cm × 555 cm) for 5 min and movement was tracked using an overhead camera and computer software (EthoVision, Noldus Information Technology, Wageningen, Netherlands). General activity levels were determined by assessing the total distance traveled. The amount of time spent along the margin of the open field as opposed to the center was taken as a measure of anxiety. The margin was defined as a 3-cm wide strip around the outside of the box.
Grip Strength
Forelimb grip strength was measured using a Chatillon force gauge (Ametek, Largo, FL). Animals were placed so their forepaws were on a wire grid connected to the force gauge. Animals were then pulled away from the wire grid, while the force meter recorded the maximum force exerted on the wire grid. Animals were given three consecutive trials, and the maximum force was taken as a measure of grip strength.
Rotarod
Agility and balance were tested using a Rotamex® rotarod device (Columbus Instruments, Columbus, OH). Animals were given two days of training, in which they were placed on the 3.5 inch rotarod turning at a rate of 4 rpm. Animals falling in less than 30 s were placed immediately back on the drum for another trial with a maximum of three trials and a time limit of 60 s. Two days after the second training session, baseline performance was assessed. Animals falling prior to 60 s were immediately given a second trial, and trials were a maximum of 300 s in duration.
Statistics
For baseline assessments, one-way ANOVA with age as a factor was used. In cases of unequal variance and non-normal data, a Kruskal-Wallis one way analysis of variance on ranks was performed. For drug administration and withdrawal data, primary statistical analysis consisted of separate two-way repeated measures ANOVA comparing age and treatment phase within each drug group. Subsequent statistical analyses consisted of two-way ANOVA comparing age and drug group for each phase of testing (i.e. early drug, late drug, early withdrawal, and late withdrawal). Student-Newman-Keuls post-hoc tests were performed where appropriate. Differences were considered statistically significant when p-values were less than 0.05. All statistical tests were performed using SigmaStat version 3.11 (Systat Software, Inc, San Jose, CA).
Results
Baseline Characteristics
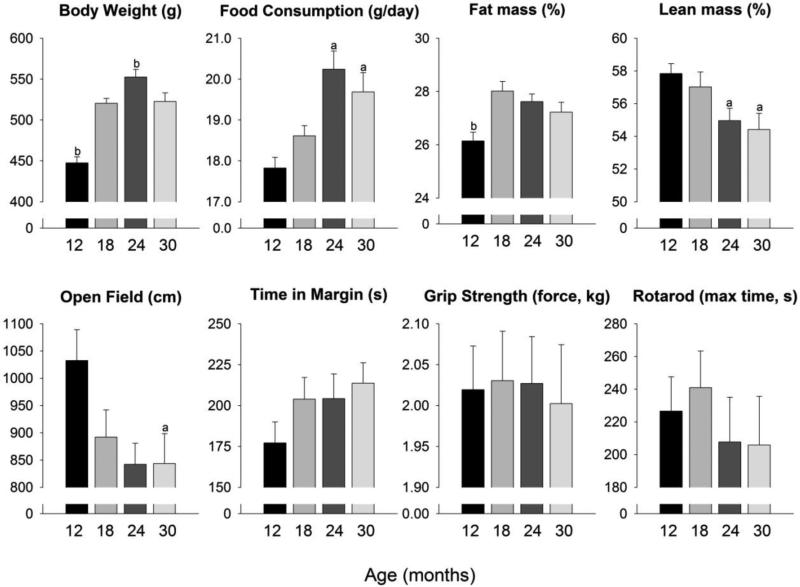
Age differences in physiology and physical performance prior to drug administration are shown in Figure 1. Food consumption at baseline differed across ages (H = 18.11, 3 d.f., p <0.001) with 12-month old animals consuming less than 24- and 30-month old animals. There were also significant age-related differences in body weight (F3, 77 = 27.94, p <0.001), such that 12-month old animals weighed less and the 24-month old rats weighed more than all other ages. Regarding body composition, 12-month old animals had significantly less fat mass than all other ages (F3, 76 = 5.64, p = 0.002) and significantly more lean mass than 24- and 30-month old animals (H = 10.87, 3 d.f., p = 0.012). During the initial assessment of physical performance measures, 12-month old animals had greater general activity than all others (F3, 75 = 3.04, p = 0.034), but there were no age differences in time spent in the margin of the activity chamber (a commonly used measure of anxiety), grip strength, or rotarod time.
Figure 1. Baseline measures.
Assessments made before implantation of osmotic pumps across the four age groups. Data are presented as means ± SEM. a p < 0.05 compared with 12 month old animals, b p < 0.05 compared with all other ages.
Food Consumption
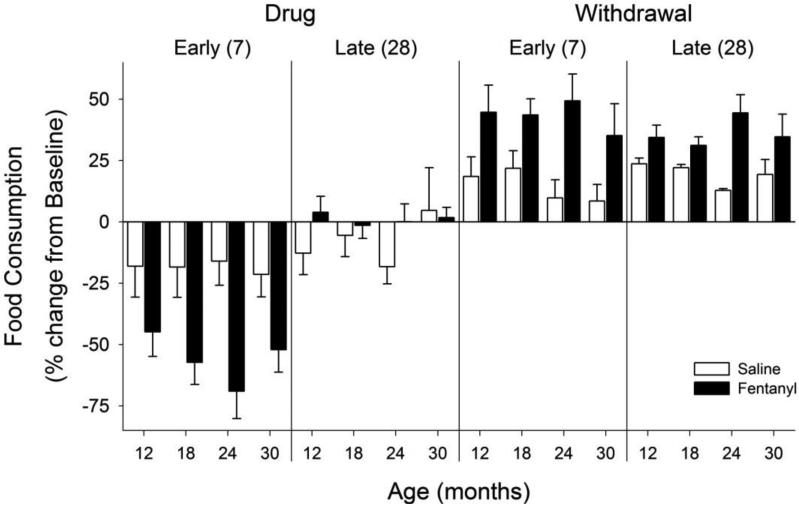
Food consumption was altered in fentanyl-treated animals (Figure 2; F4, 93 = 85.01, p = <0.001). During early chronic drug administration, food consumption was significantly decreased relative to baseline, and tolerance appeared to develop to this effect as food consumption returned to baseline levels over the 4 weeks of drug administration. During both early and late withdrawal periods, food consumption was above baseline in all age groups. This change in food consumption was also seen when the fentanyl-treated rats were directly compared to saline-treated rats during early drug and withdrawal, and following four weeks of withdrawal.
Figure 2. Food consumption.
Changes in food consumption during fentanyl/saline administration (“Drug”) and “Withdrawal”. Each phase is divided into an “Early” and “Late” stage, and the number in parentheses represents the number of days following the minipump implantation or removal that the measure was taken. Data are presented as percent change from baseline (mean ± SEM) for each age group. Note that fentanyl decreased food consumption across ages during early assessments, and increased food consumption throughout withdrawal.
Body Weight
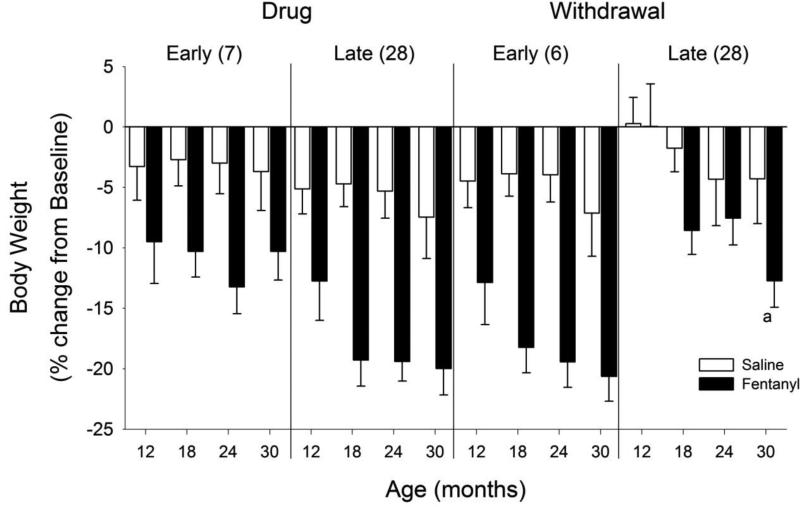
Figure 3 shows a significant interaction of age and phase for the fentanyl-treated animals (F12, 102 = 4.14, p <0.001). Fentanyl administration decreased body weights significantly during early and late drug administration and during early withdrawal, and this effect was greatest in the older animals. During late withdrawal, body weight for 12-month old animals had returned to baseline, but other ages had not fully recovered, with the body weight for 30-month old animals being significantly less than 12-month old animals. Across all phases, direct comparison of fentanyl versus saline treatment revealed lower body weights in fentanyl-treated animals.
Figure 3. Body weight.
Changes in body weight during fentanyl/saline administration and withdrawal. Other details as in Figure 2. Note that fentanyl resulted in decreased body weight across ages during the Early and Late drug administration, and during Early withdrawal. By 28 days of withdrawal, the youngest animals returned to baseline levels. a p < 0.05 compared with 12 month old animals.
Body Composition
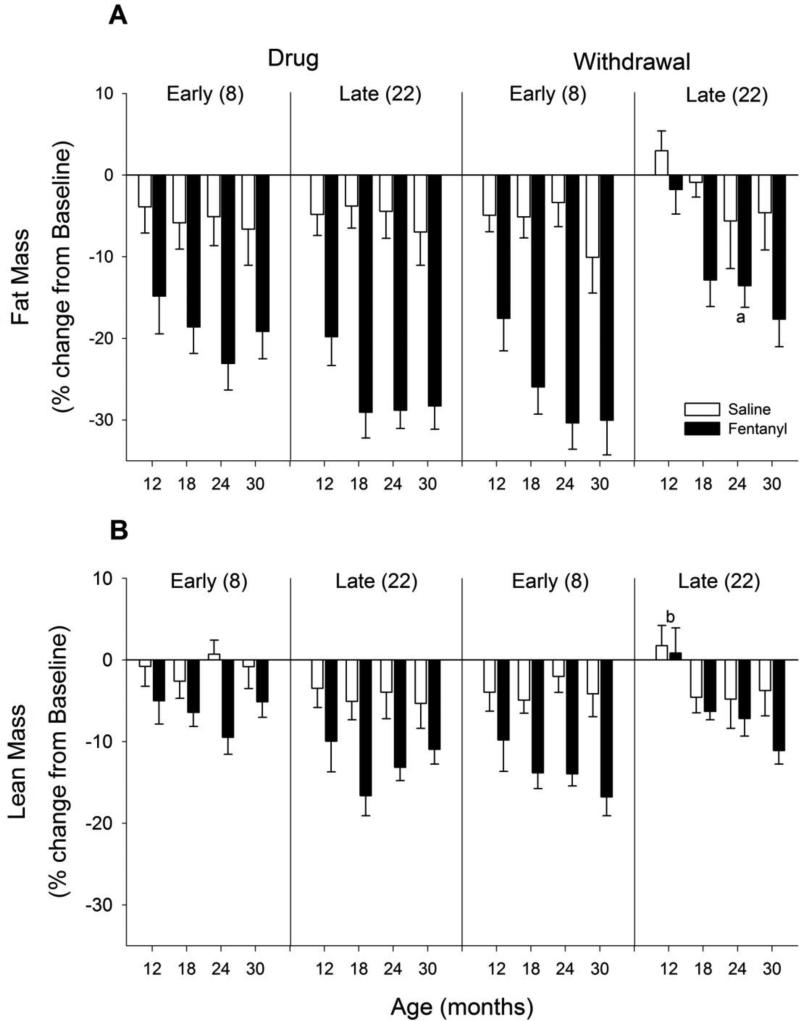
Using TD-NMR, decreases in both fat mass and lean mass during drug administration and withdrawal were observed (Figure 4). Similar to body weight, there was an interaction of age and phase for both fat and lean mass in fentanyl-treated animals (fat: F12, 104 = 2.18, p = 0.018; lean: F12, 104 = 2.50, p = 0.006). Fentanyl-treated animals lost both fat and lean mass during early and late drug administration as well as during early withdrawal. During late withdrawal, fat and lean mass had returned to baseline levels for 12-month old animals, but older animals were slower to recover, with 30-month old animals showing significantly lower fat and lean mass levels relative to 12-month old animals. During all phases, fentanyl-treated animals had significantly lower levels of fat and lean mass than saline-treated animals. In a direct comparison of tissue type (fat versus lean) and across ages, fentanyl had a greater effect on fat mass than lean mass (all p values < 0.05).
Figure 4. Body composition.
Changes in (A) fat mass and (B) lean/muscle mass during fentanyl/saline administration and withdrawal. Other details as in Figure 2. Note that fentanyl resulted in decreased fat and muscle mass across ages during Early and Late drug administration, and during Early withdrawal. By 22 days of withdrawal, the youngest animals returned to baseline levels. a p < 0.05 compared with 12 month old animals, b p < 0.05 compared with all other ages.
General Activity
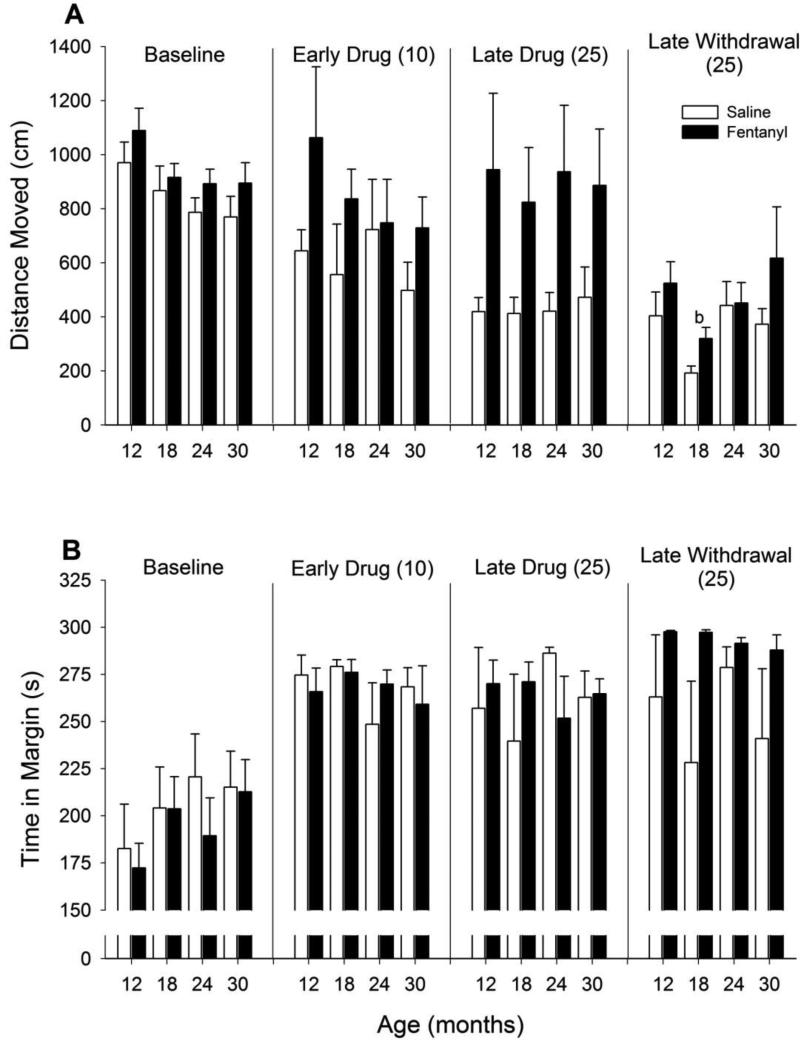
In saline-treated animals, there was a habituation-like process with a decrease in activity across phases (Figure 5A; F3, 94 = 23.81, p = <0.001). In fentanyl-treated animals, only activity during the late withdrawal period was significantly decreased relative to baseline (F3, 81 = 8.88, p = <0.001). Comparison of fentanyl versus saline showed that saline-treated animals had significantly lower levels of activity during early drug, late drug, and late withdrawal phases. The amount of time spent along the edges of the open field device is often taken as a measure of anxiety. There were little differences in this measure across ages or phases with the exception of a long-lasting increase (i.e. during late withdrawal) in the fentanyl-treated animals (Figure 5B; F1, 54 = 4.46, p = 0.039).
Figure 5. Open field activity.
Changes in (A) total distance traveled and (B) time spent in the margin. Other details as in Figure 2. Note that saline-treated animals showed decreased activity over repeated testing (i.e. habituation), whereas fentanyl resulted in heightened levels of activity throughout drug administration. Following 24 days of drug withdrawal, fentanyl-treated animals spend essentially the entire session in the margin, a finding consistent with increased levels of anxiety. b p < 0.05 compared with all other ages.
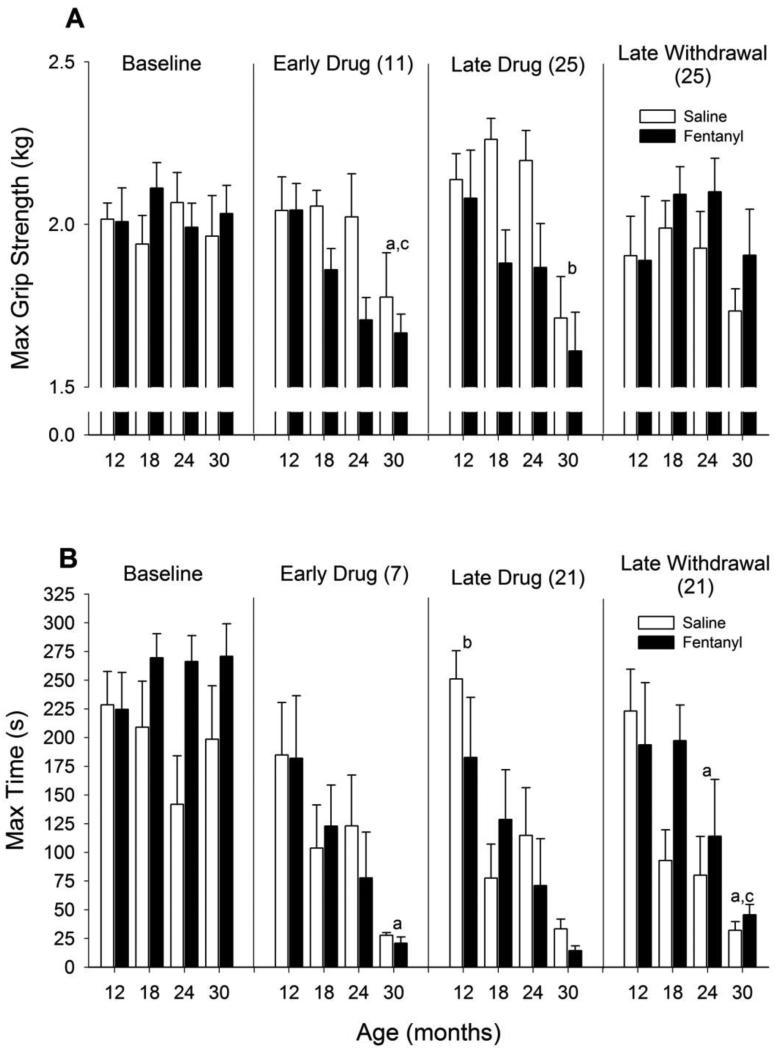
Grip Strength
During acute drug administration (Figure 6A), age had a significant impact on maximum grip strength similar to previous studies (Carter et al., 2004; Forester & Lal, 1999) with grip strength decreasing with age (F3, 59 = 4.19, p = 0.009). Fentanyl administration decreased grip strength across ages, but this effect was resolved during late withdrawal (early drug: F1, 59 = 5.28, p = 0.025; late drug: F1, 56 = 7.907, p = 0.007).
Figure 6. Physical performance measures.
Changes in (A) maximum grip strength and (B) time spent on rotarod. Other details as in Figure 2. Note decreases in grip strength during fentanyl administration although this decrease does not persist after 25 days of withdrawal. a p < 0.05 compared with 12-month old animals, b p < 0.05 compared with all other ages, c p < 0.05 compared with 18-month old animals. For rotarod performance, there were decreases in time with increasing age regardless of drug treatment. a p < 0.05 compared with 12-month old animals, b p < 0.05 compared with all other ages, c p < 0.05 compared with 18-month old animals.
Rotarod
During all treatment phases (Figure 6B), there was a prominent age effect with older animals having impaired rotarod performance relative to younger animals (early drug: F3, 55 = 5.20, p = 0.003; late drug: F3, 52 = 9.11, p = <0.001; late withdrawal: F3, 50 = 7.11, p = <0.001). However, there was no effect of fentanyl administration on rotarod performance during any phase.
Discussion
There is growing concern over the increasing use of opioids to treat chronic pain in the elderly primarily because of the potential increased sensitivity to the adverse side effects (Benyamin et al., 2008; Hutchinson et al., 2007; Wilder-Smith, 2005). Unfortunately, an increased risk to these effects is not well documented (Bernabei et al., 1998; Fine, 2004; Pergolizzi et al., 2008). The purpose of this study was to assess differential effects of chronic fentanyl administration across ages on various measures of physiology and behavior. Overall, the most profound effects of fentanyl administration were on food consumption, body weight, and body composition. Fentanyl administration resulted in decreases in food consumption and a long-term decrease in body weight, primarily due to decreases in fat mass. Even after a month of withdrawal from fentanyl administration, only the youngest rats had returned to baseline body weight. Given the literature demonstrating the difficulty of regaining unexpected or unintentional body weight loss and the detrimental functional consequences of such loss in older persons (e.g. Lee et al., 2005; Locher et al., 2007; Miller & Wolfe, 2008; Ritchie et al., 2008), the current findings suggest that body weight should be monitored not only during chronic drug administration but for extended periods following cessation of drug treatment.
Physiological consequences of chronic opioid administration
There is a large literature documenting the effects of opioids on food consumption (e.g. Bodnar, 2004), and in general it is known that acute administration of opioid agonists increases food consumption in rats (Sanger & McCarthy, 1981) while opioid antagonists decrease food consumption (Glass et al., 1999; McLaughlin & Baile, 1983; Yuan et al., 2009). However, studies looking at chronic administration of morphine show decreases in food intake and body weight (Binsack et al., 2006; Levine et al., 1988; Li et al., 2010), while chronic opioid antagonist administration increases food consumption and weight gain (Chen et al., 2004). As hypothesized, chronic fentanyl administration resulted in a decreased food consumption within the first week, and animals became tolerant to this effect as food consumption had returned to baseline after four weeks of fentanyl administration. During withdrawal, fentanyl-treated animals showed an increase in food consumption. Clinically, these data suggest that at the beginning of chronic opioid administration, it is important to make sure that patients are receiving adequate food intake even though these effects are not persistent through the duration of opioid administration or withdrawal.
One predictable consequence of decreases in food consumption is a change in body weight. Of importance is the fact that while obesity leads to major health risks in younger populations, studies have shown that in individuals 60 years old and older, being underweight has a greater risk of mortality and disability than being overweight or even obese (Flegal et al., 2005; Marcell, 2003; Miller & Wolfe, 2008; Paddon-Jones et al., 2008). In the current study, fentanyl administration resulted in a decrease in body weight for all ages throughout the 28 days of drug administration. Using TD-NMR, body composition changes were followed through drug administration and withdrawal, and the overall pattern of decreases in fat and lean (muscle) mass followed a similar course as the changes in body weight. Although food consumption returned to baseline levels by the end of drug administration and increased through one week of withdrawal, body weight (and both fat and lean mass components) remained decreased through withdrawal. Only after one month of withdrawal had these measures started to return to baseline, and the older animals showed less recovery than the younger animals. In general, the effect of fentanyl administration on fat mass relative to lean mass was of a greater magnitude and longer lasting. The fact that only the youngest animals fully regain their body weight at the end of withdrawal even though there are no age differences in food consumption suggests that the decrease in food consumption is not wholly responsible for the decrease in body weight. However, although older individuals may take longer to regain body weight after ending chronic fentanyl administration, lean mass is recovered more quickly than fat mass, which is an important factor in the health of aged individuals (Marcell, 2003).
Behavioral consequences of chronic opioid administration
Given the increasing use of fentanyl, the high prevalence of chronic pain, and the aging of the population, it is critical to identify some of the functional consequences of chronic fentanyl administration across a range of ages. This was especially important as we identified profound changes in body weight and composition that were longer-lasting in the oldest animals and could lead to decreased strength and functional independence, and eventually increased frailty, disability, and mortality.
Similar to previous studies, there were age-related differences in overall activity levels assessed in an open field at baseline, and during the initial drug administration phase declines were observed in rotarod and grip strength (Carter et al., 2004; Forster & Lal, 1999). Fentanyl appeared to have effects on both activity levels (increases) and grip strength (decreases) at the late drug time point, and these effects were not maintained into the late withdrawal phase. These findings are consistent with the literature showing that repeated administration of mu opioid agonists (including fentanyl and morphine) can result in long-lasting increases in locomotor activity levels in rats (e.g. Khallouk-Bousselmame & Costentin, 1994; Powell & Holtzman, 2001; Rauhala et al., 1995; Trujillo et al., 2004). Clinically, extended fentanyl administration increases activity, although it is unclear if this is a direct effect of fentanyl or a by-product of mitigation of chronic pain in these patients (Agarwal et al., 2007). Age-related decreases in rotarod performance were maintained throughout all phases of drug/saline administration, however fentanyl did not influence performance. These findings suggest that while chronic fentanyl administration has lasting effects on physiological measures, this does not necessarily translate into decrements observed in these behavioral measures. Importantly, these data provide information suggesting that strong opioids such as fentanyl do not necessarily result in more dramatic and potentially dangerous adverse behavioral side effects with increases in age.
Implications
The relative age of the population is increasing, there is a high prevalence of age-related chronic pain in this population, and opioids are being used more commonly for a greater number of clinical conditions. The potential adverse physiological and behavioral outcomes of chronic opioid administration in aged populations are relatively unknown. The present study characterized some differential consequences of chronic fentanyl administration to rats of various ages. Chronic opioid administration had effects on both physiological measures (body weight, body composition, food consumption) and behavioral tests (grip strength and open field). Lasting effects were only observed in these physiological measures. Fentanyl administration decreased body weight (both fat mass and lean, muscle mass) across all ages, but older animals took longer to recover body weight following cessation of fentanyl administration. This slow recovery in body weight occurs even in the face of apparent tolerance to the anorectic effects as food consumption levels returned to then surpassed baseline levels of intake. On the positive side, these dramatic changes in body weight and consumption did not necessarily translate into long-lasting functional deficits assessed using a number of behavioral measures. Taken together, these data suggest that chronic opioid administration to aged individuals can have dramatic and long-lasting effects that should be routinely assessed even following prolonged periods of withdrawal from the drug.
Acknowledgements
This research was primarily supported by a grant from the NIH (R21DA023022 to DM). We would like to thank Alex Bibbey, Greg Foremny, Gebreyes Kassu, and Sheila Quintana for assistance with data collection. CSC was supported by US Public Health grant R01AG24526. JDM was supported by a T32 training grant (AG00196) and the University of Florida Alumni Fellowship. This article was used in partial fulfillment for the requirements of the doctoral degree at the University of Florida for the first author. Additional support was provided by the University of Florida Claude D. Pepper Older Americans Independence Center grant P30AG028740.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Polydefkis M, Block B, Haythornwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med. 2007;8:554–562. doi: 10.1111/j.1526-4637.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Alturi S, Boswell MV, Hansen HC, Trescot AM, Singh V, Jordan AE. Guidelines for the use of controlled substances in the management of chronic pain. Pain Physician. 2003;6:233–257. [PubMed] [Google Scholar]
- Auret K, Schug SA. Underutilisation of opioids in elderly patients with chronic pain: approaches to correcting the problem. Drugs Aging. 2005;22:641–654. doi: 10.2165/00002512-200522080-00002. [DOI] [PubMed] [Google Scholar]
- Bell JS, Klaukka T, Ahonen J, Hartikainen S. National utilization of transdermal fentanyl among community-dwelling older people in Finland. Am. J. Geriatr. Pharamacother. 2009;7:355–361. doi: 10.1016/j.amjopharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complication and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Bernabei R, Gambassi G, Lapane K, Landi F, Gatsonis C, Dulop R, et al. Management of pain in elderly patients with cancer. SAGE study group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y, Gross DP, Haykowsky M, Rashiq S. Effect of opioid administration on cardiorespiratory and muscle oxygenation during lifting in chronic back pain patients. Eur. J. Appl. Physiol. 2010;109:241–250. doi: 10.1007/s00421-009-1332-y. [DOI] [PubMed] [Google Scholar]
- Binsack R, Zheng ML, Zhang ZS, Yang L, Zhu YP. Chronic morphine drinking establishes morphine tolerance, but not addiction in Wistar rats. J Zhejiang Univ Sci B. 2006;7:892–898. doi: 10.1631/jzus.2006.B0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Brixner DI, Oderda GM, Roland CL, Rublee DA. Opioid expenditures and utilization in the Medicaid system. J. Pain Palliat. Care Pharmacother. 2006;20:5–13. [PubMed] [Google Scholar]
- Byas-Smith MG, Chapman SL, Reed B, Cotsonis G. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin. J. Pain. 2005;21:345–352. doi: 10.1097/01.ajp.0000125244.29279.c1. [DOI] [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang RR, Shen CP, MacNeil DJ, Fong TM. Chronic administration of nalmefene leads to increased food intake and body weight gain in mice. Eur. J Pharmacol. 2004;495:63–66. doi: 10.1016/j.ejphar.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Solomon DH, Roth CP, Chang JT, MacLean CH, Ferrell BA, et al. The quality of medical care provided to vulnerable older patients with chronic pain. J Am. Geriatr Soc. 2004;52:756–761. doi: 10.1111/j.1532-5415.2004.52214.x. [DOI] [PubMed] [Google Scholar]
- de Leon-Casasola OA. Current developments in opioid therapy for management of cancer pain. Clin. J. Pain. 2008;24:S3–7. doi: 10.1097/AJP.0b013e31816b589f. [DOI] [PubMed] [Google Scholar]
- Delgado-Guay MO, Bruera E. Management of pain in the older person with cancer. Part 2: treatment options. Oncology (Williston Park, N.Y.) 2008;22:148–152. [PubMed] [Google Scholar]
- Donald IP, Foy C. A longitudinal study of joint pain in older people. Rheumatology (Oxford) 2004;43:1256–1260. doi: 10.1093/rheumatology/keh298. [DOI] [PubMed] [Google Scholar]
- Fine PG. Opioid analgesic drugs in older people. Clin. Geriatr. Med. 2001;17:479–487. doi: 10.1016/s0749-0690(05)70081-1. [DOI] [PubMed] [Google Scholar]
- Fine PG. Pharmacological management of persistent pain in older patients. Clin J Pain. 2004;20:220–226. doi: 10.1097/00002508-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol. Aging. 1999;20:167–176. doi: 10.1016/s0197-4580(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ. 1999;160:329–333. [PMC free article] [PubMed] [Google Scholar]
- Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Garcia del Pozo J, Carvajal A, Viloria JM, Velasco A, Garcia del Pozo V. Trends in the consumption of opioid analgesics in Spain. Higher increases as fentanyl replaces morphine. Eur. J. Clin. Pharmacol. 2008;64:411–415. doi: 10.1007/s00228-007-0419-9. [DOI] [PubMed] [Google Scholar]
- Gianni W, Madaio RA, Di Cioccio L, D'Amico F, Policicchio D, Postacchini D, et al. Prevalence of pain in elderly hospitalized patients. Arch Gerontol Geriatr. doi: 10.1016/j.archger.2009.11.016. In Press. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Grape S, Schug SA, Lauer S, Schug BS. Formulations of fentanyl for the management of pain. Drugs. 2010;70:57–72. doi: 10.2165/11531740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin. Geriatr. Med. 2001;17:417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- Herndon CM, Jackson KC, II, Hallin PA. Management of opioid-induced gastrointestinal effects in patients receiving palliative care. Pharmacotherapy. 2002;22:240–250. doi: 10.1592/phco.22.3.240.33552. [DOI] [PubMed] [Google Scholar]
- Hong JY, Jee YS, Jeong HJ, Song Y, Kil HK. Effects of epidural fentanyl on speed and quality of block for emergency cesarean section in extending continuous epidural labor analgesia using ropivacaine and fentanyl. J. Korean Med. Sci. 2010;25:287–292. doi: 10.3346/jkms.2010.25.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson K, Moreland AM, de C Williams AC, Weinman J, Horne R. Exploring beliefs and practice of opioid prescribing for persistent non-cancer pain by general practitioners. Eur. J. Pain. 2007;11:93–98. doi: 10.1016/j.ejpain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research . Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, D.C.: 1996. [Google Scholar]
- Khallouk-Bousselmame R, Costentin J. Locomotor and analgesic effects of morphine and acetorphan in rats chronically treated with morphine or thiorphan. Eur Neuropsychopharmacol. 1994;4:137–143. doi: 10.1016/0924-977x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kritchevsky SB, Harris TB, Tylavsky F, Rubin SM, Newman AB. Short-term weight changes in community-dwelling older adults: the Health, Aging, and Body Composition Weight Change Substudy. Am. J. Clin. Nutr. 2005;82:644–650. doi: 10.1093/ajcn.82.3.644. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ, Gosnell BA, Krahn DD, Brown DM, et al. Effect of morphine and nalmefene on energy balance in diabetic and non-diabetic rats. Pharmacol. Biochem. Behav. 1988;29:495–500. doi: 10.1016/0091-3057(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Li P, Maguma HT, Thayne K, David B, Taylor DA. Correlation of the time course of development and decay of tolerance to morphine with alterations in sodium pump protein isoform abundance. Biochem. Pharmacol. 2010;79:1015–1024. doi: 10.1016/j.bcp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin. J. Pain. 2007;23:799–803. doi: 10.1097/AJP.0b013e3181565cf1. [DOI] [PubMed] [Google Scholar]
- Locher JL, Roth DL, Ritchie CS, Cox K, Sawyer P, Bodner EV, Allman RM. Body mass index, weight loss, and mortality in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1389–1392. doi: 10.1093/gerona/62.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- McLaughlin CL, Baile CA. Nalmefene decreases meal size, food and water intake and weight gain in Zucker rats. Pharmacol. Biochem. Behav. 1983;19:235–240. doi: 10.1016/0091-3057(83)90045-x. [DOI] [PubMed] [Google Scholar]
- McNeill JA, Sherwood GD, Starck PL. The hidden error of mismanaged pain: a systems approach. J. Pain Symptom Manage. 2004;28:47–58. doi: 10.1016/j.jpainsymman.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miller SL, Wolfe RR. The danger of weight loss in the elderly. J. Nutr. Health Aging. 2008;12:487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- Morgan D, Carter CS, DuPree JP, Yezierski RP, Vierck CJ. Evaluation of prescription opioids using operant-based pain measures in rats. Exp. Clin. Psychopharmacol. 2008;16:367–375. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC. The functional observational battery in adult and developing rats. Neurotoxicology. 2000;21:989–996. [PubMed] [Google Scholar]
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- Potter J, Higginson IJ. Pain experienced by lung cancer patients: a review of prevalence, causes, and pathophysiology. Lung Cancer. 2004;43:247–257. doi: 10.1016/j.lungcan.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Powell KR, Holtzman SG. Parametric evaluation of the development of sensitization to the effects of morphine on locomotor activity. Drug Alcohol Depend. 2001;62:83–90. doi: 10.1016/s0376-8716(00)00167-8. [DOI] [PubMed] [Google Scholar]
- Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. J. Natl. Cancer Inst. Monographs. 2004;(32):150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- Rauck R, North J, Gever LN, Tagarro I, Finn AL. Fentanyl buccal soluble film (FBSF) for breakthrough pain in patients with cancer: a randomized, double-blind, placebo-controlled study. Ann. Oncol. 2010;21:1308–1314. doi: 10.1093/annonc/mdp541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala P, Idänpään-Heikkilä JJ, Tuominen RK, Männistö PT. Differential disappearance of tolerance to thermal, hormonal, and locomotor effects of morphine in the male rat. Eur. J. Pharmacol. 1995;285:69–77. doi: 10.1016/0014-2999(95)00392-x. [DOI] [PubMed] [Google Scholar]
- Ritchie CS, Locher JL, Roth DL, McVie T, Sawyer P, Allman R. Unintentional weight loss predicts decline in activities of daily living function and life-space mobility over 4 years among community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:67–75. doi: 10.1093/gerona/63.1.67. [DOI] [PubMed] [Google Scholar]
- Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin. J. Pain. 2005;21:513–523. doi: 10.1097/01.ajp.0000146217.31780.ef. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, McCarthy PS. Increased food and water intake produced in rats by opiate receptor agonists. Psychopharmacology. 1981;74:217–220. doi: 10.1007/BF00427097. [DOI] [PubMed] [Google Scholar]
- Scudds RJ, Robertson JM. Pain factors associated with physical disability in a sample of community-dwelling senior citizens. J. Gerontol. A. Biol. Sci. Med. Sci. 2000;55:M393–M399. doi: 10.1093/gerona/55.7.m393. [DOI] [PubMed] [Google Scholar]
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am. Fam. Physician. 2006;74:1347–1354. [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Thomason TE, McCune JS, Bernard SA, Winer EP, Tremont S, Lindley CM. Cancer pain survey: patient-centered issues in control. J. Pain Symptom Manage. 1998;15:275–284. doi: 10.1016/s0885-3924(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Datta S, Lee M, Hansen H. Opioid Pharmacology. Pain Physician. 2008;11:S133–S153. [PubMed] [Google Scholar]
- Trujillo KA, Kubota KS, Warmoth KP. Continuous administration of opioids produces locomotor sensitization. Pharmacol. Biochem. Behav. 2004;79:661–669. doi: 10.1016/j.pbb.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith OH. Opioid use in the elderly. Eur. J. Pain. 2005;9:137–140. doi: 10.1016/j.ejpain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Wang CZ, Attele A, Zhang L. Methylnaltrexone reduced body weight gain in ob/ob mice. J Opioid Manag. 2009;5:213–218. doi: 10.5055/jom.2009.0023. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Griffiths PC, Berg S. Pain perceptions of the oldest old: a longitudinal study. Gerontologist. 2004;44:459–468. doi: 10.1093/geront/44.4.459. [DOI] [PubMed] [Google Scholar]