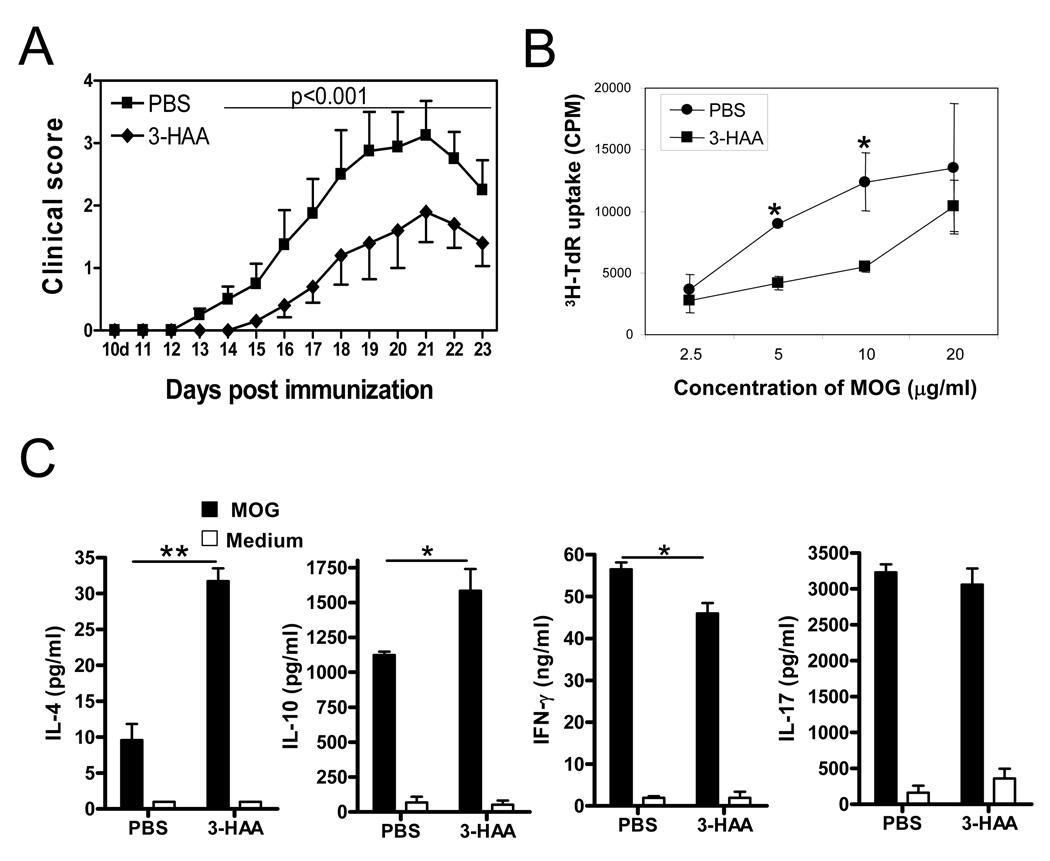
Figure 2. 3-HAA treatment ameliorates clinical severity of EAE.
C57BL/6 mice were immunized by an EAE-inducing protocol with 150 µg MOG35–55 peptide. Tested mice were injected i.p. with 200 mg/kg of 3-HAA in PBS daily, beginning on day 1 p.i. This route and dose were selected based on the study by Platten et al. (29). Control mice were given the same amount of PBS. One representative of three independent experiments is shown. A. Clinical scores were recorded daily. Results are shown as mean ± SE (n = 5). B. Spleen cells were harvested at the termination of the experiment and were stimulated by MOG35–55 peptide in vitro. Cell proliferation was assessed by 3H-TdR incorporation (mean ± SD of triplicate). p < 0.05. C. Culture supernatants from spleen cells described in B were assayed for cytokines by ELISA. Results are shown as mean ± SD of duplicates. Data are from a representative of three independent experiments. *p < 0.05; **p < 0.01.