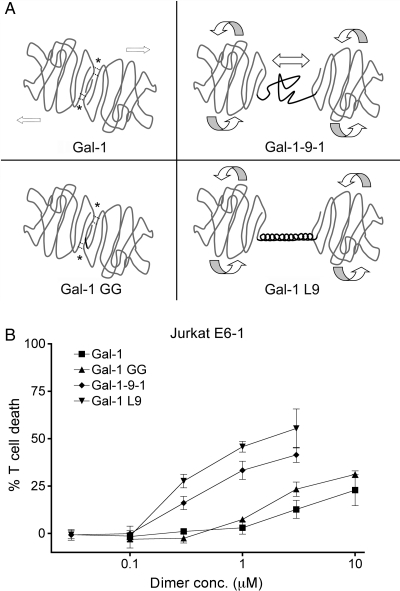
Fig. 1.
(A) Schematic of galectin-1 constructs. Upper left: galectin-1 forms homodimers via hydrophobic interactions (*). Lower left: gal-1GG has a glycine–glycine linker (black line) between the C-terminus of one CRD and the N-terminus of the second CRD. Hydrophobic interactions at the dimer interface are maintained. Upper right: gal-1-9-1 has two galectin-1 CRDs joined by the galectin-9 short random-coil linker region (black line), which would allow the rotation and lateral movement of the CRDs. Lower right: gal-1L9 has two galectin-1 CRDs joined by the bacterial ribosomal L9 rigid α-helix peptide (black line). Gal-1L9 would allow the rotation of CRDs, but not lateral movement. (B) Potency of galectin-1 constructs. Galectins with long linker regions are more potent in T cell death assays. Jurkat E6-1 T cells were incubated with the indicated dimer concentration of galectin-1 (filled square), gal-1GG (filled triangle), gal-1-9-1 (filled diamond), gal-1L9 (filled inverted triangle) or buffer control for 6 h. Data represent mean ± SD of triplicate samples.