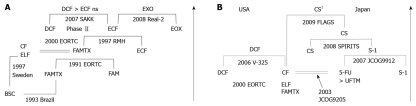
Figure 1.
Phase III trials are the most successful for far advanced gastric cancer in Europe (A) and in both United States and Japan (B). Arrows show chronological direction. BSC: Best supportive care; FAMTX: 5-FU/Adriamycin/Methotrexate; CF: Cisplatin/5-FU; ELF: Etoposide/Leucovorin/5-FU; FAM: 5-FU/Adriamycin/Mitomycin-C; ECF: Epirubicin/Cisplatin/5-FU; EOX: Epirubicin/Oxaliplatin/Capecitabine; DCF: Docetaxel/Cisplatin/5-FU; EORTC: European Organization for Research and Treatment of Cancer; RMH: Royal Marsden Hospital; SAKK: Swiss Group for Clinical Cancer Research; ns: Not significant. UFTM: UFT/Mitomycin-C; CS: S-1/Cisplatin; 1CS regimens in the FLAGS study used 25 mg/m2 of S-1 differently from the SPILITS trial (40 mg/m2 of S-1).