Figure 2.
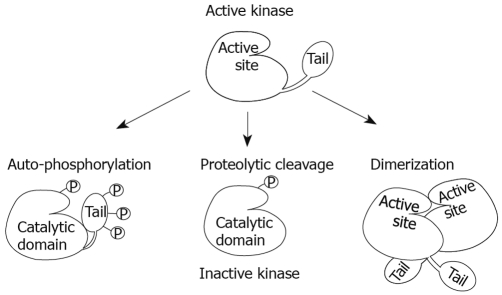
Models of CKIepsilon/δ regulation. CKIepsilon/δ auto-phosphorylation at their C-terminal tail inhibits their kinase activity through a conformational change that places the tail over the active site, therefore blocking it from potential substrates. Dephosphorylation by phosphatases is the most accepted mechanism for their activation in vivo. In addition, removal of the tail by proteolytic cleavage or mutations has been used in vitro to activate these kinases; however, actual removal of the tail in vivo may have additional effects on proper function. Finally, CKIepsilon/δ dimerization at the active site could inactivate these kinases by physically blocking substrates from entering this region.