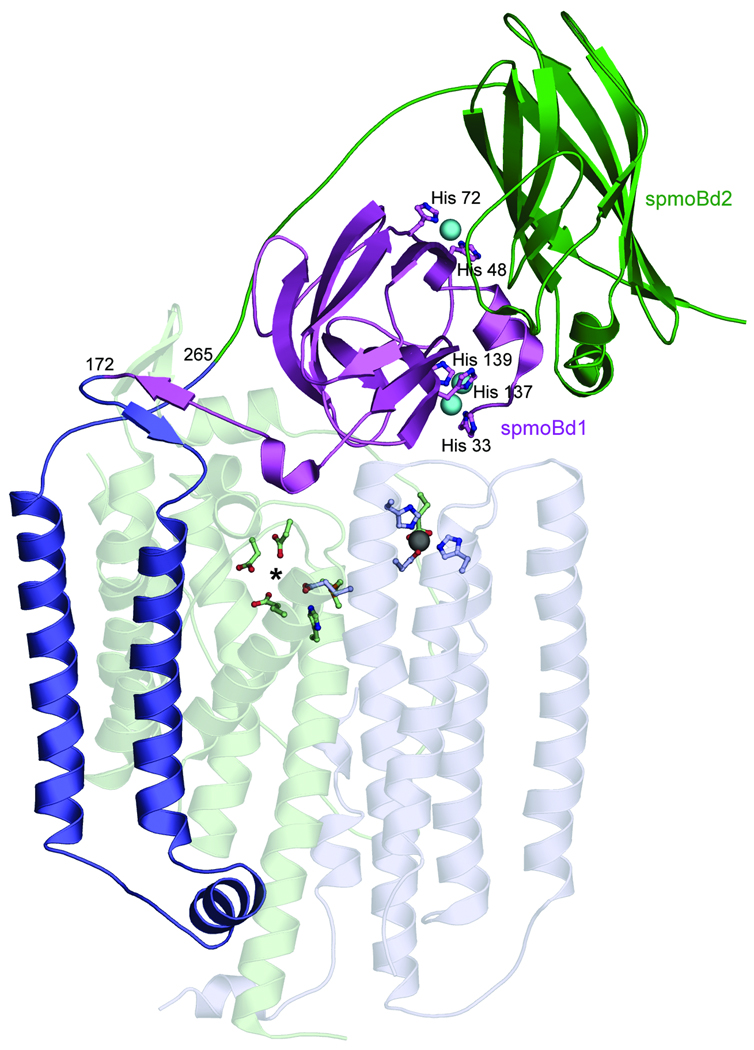
Figure 1. Structure of M. capsulatus (Bath) pMMO protomer.
(PDB accession code 1YEW). The N-terminal cupredoxin domain of pmoB (spmoBd1) is shown in purple, the C-terminal cupredoxin domain of pmoB (spmoBd2) is shown in green, and the two transmembrane helices are shown in blue. In the recombinant spmoB protein, spmoBd1 and spmoBd2 are connected by a GKLGGG sequence linking residues 172 and 265 instead of the two transmembrane helices. Copper ions are shown as cyan spheres and ligands are shown as ball-and-stick representations. The pmoA (transparent light green) and pmoC (transparent light blue) subunits are composed of transmembrane helices. The location of the zinc ion (gray sphere) has been proposed to house a diiron center. A hydrophilic patch of residues marked with an asterisk is the site of a proposed tricopper center.