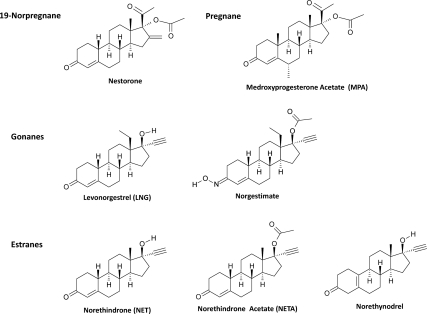
Figure 1.
Chemical structures of clinically relevant progestins. Progestins are derived from four tetracyclic structures: 19-norprogesterone, pregnane, estrane, and gonane. Although all three structures bear four rings, identified as A, B, C, and D, the estrane structure differs from the pregnane structure by lacking the C-19 angular methyl radical between rings A and B. Gonane structure lacks both the C-18 and C-19 angular methyl radicals and bears an ethyl group between rings C and D at C-13. Removal of C-19 methyl radical and addition of C-13 ethyl radical significantly enhances the progestational activities. The seven representative progestins were the 19-norprogesterone, Nestorone; the pregnane, MPA; the gonanes, leveonorgestrel and NGM; and the estranes, NET, NETA, and norethynodrel.