Abstract
Background
Clinical outcomes are worse for heart failure (HF) patients presenting with symptoms of depression. Sympathetically modulated immune dysregulation associated with depression may be one mechanism leading to worse prognosis.
Objectives
We sought to determine whether depressive symptoms are related to alterations in sensitivity of peripheral blood mononuclear cells (PBMC) to β-adrenergic agonists in HF patients by measuring in vitro chemotaxis (CTX) to isoproterenol (ISO) at rest and following acute exercise in HF patients and controls.
Methods
80 HF patients and 44 controls (mean age ± SEM: 56.4 ± 1.3 years) completed the Beck Depression Inventory (BDI) and a 15 minute mild graded exercise task on a stationary bicycle. Exercise intensity was kept relative to fitness levels for all participants by gradually increasing resistance to reach a Borg scale subjective rating of 12 –13, “somewhat hard”. Plasma norepinephrine (NE) and epinephrine (EPI) levels were measured in plasma before and after exercise. Chemotaxis to ISO (CTX-I) was determined by measuring in vitro PBMC migration through a modified Boyden chamber.
Results
In HF patients, depressive symptom severity was associated with greater CTX following exercise (p = .001). Higher resting NE in HF patients was also associated with increased CTX to exercise (p = .03).
Conclusion
HF patients with higher depression symptoms and NE exhibited increased PBMC CTX-I to mild exercise, suggesting greater β-adrenergic sensitivity. Increased immune migration in HF patients having elevated depression symptoms could be associated with cardiac remodelling and HF disease progression.
Keywords: adrenergic, chemotaxis, depression, heart failure, exercise
INTRODUCTION
The prevalence of depression ranges from 20% to 45% in symptomatic heart failure (HF) patients, and corresponds with significantly greater morbidity and mortality(1). However, the biobehavioral pathways linking depression with adverse outcomes in HF are unclear. One potential mechanism is depression-associated dysregulation of neuroendocrine modulation of immune responses to stress and exercise (2), which may be injurious to the cardiovascular system (3) leading to worse prognosis in HF (4,5).
Autonomic innervation and regulation of the immune system is well recognized (e.g. (6,7)). Sympathetic activation during stress and exercise elicits the release of leukocytes from the spleen, lymph nodes and blood vessel sub-endothelia into the blood stream (8,9). These immune cells express greater surface β-adrenergic receptor (β-AR) density and sensitivity (9–11). The endogenous β–adrenergic neurohormones norepinephrine (NE) and epinephrine (EPI) can thereby regulate immune mobility, as powerful chemoattractants (6,12). Additional inflammatory markers also respond to exercise related increases in catecholamines in coronary artery disease patients compared with controls (13).
Depression is associated with modified sympathetic/neurohormonal activation to stress and exercise, including NE and EPI (14). Thus, depression may alter immune responses to stress and exercise through changes in neuroendocrine-immune interactions. Indeed, depression is associated with increased leukocyte sensitivity to stress hormones (15), and greater pro-inflammatory responses to stress and physical exertion (2,3). Our research suggests that HF patients with elevated depression symptoms also exhibit augmented immune migration processes to exercise (16,17). While the clinical relevance of these findings remains to be elucidated, excessive leukocyte mobilization can elicit leukocyte infiltration into myocardial interstitium, which can underlie injury to the cardiovascular system (4,5).
In light of prior research, the primary objective of the present study was to investigate the relationship between depression symptoms in HF patients and leukocyte mobility toward a β-adrenergic agonist in response to physical exertion, as a model to explore changes in immune cell sensitivity to neuroendocrine modulation in this group. This was done by assessing depression symptoms and in vitro chemotaxis of peripheral blood mononuclear cells (PBMC) to isoproterenol (CTX-I) at rest and after acute exercise, comparing HF patients and non-HF controls. Furthermore, the influence of endogenous sympathetic activity on these relationships was explored. Determination of a link between depression and neuroimmune dysregulation in HF patients may suggest one mechanism that leads to worse HF outcomes.
METHODS
Disclosures
There are no conflicts of interest to disclose.
Study participants
Included in the study were 124 subjects (80 HF patients and 44 non-HF controls) assessed for in vitro CTX-I, depression symptoms, physical function and demographic variables from years 2005 to 2009. Patients were recruited from the San Diego Veterans Affairs Medical Center and the University of California, San Diego Medical Center as part of a larger study on the effects of depression on neuroimmunity in HF. Control subjects were recruited through advertisements and word of mouth referrals.
Inclusion criteria for all subjects were ages 30 – 85 years, blood pressure < 180/110 mm Hg, and men and women of all ethnicities and races. HF patients were NYHA classes II through IV, symptoms of HF for at least 3 months optimally treated with β-blockers, diuretics and ACE inhibitors, and systolic dysfunction defined by an ejection fraction ≤ 45% or diastolic dysfunction with preserved ejection fraction. Left ventricular ejection fraction (LVEF) was assessed by echocardiography. A six-minute walk-test assessed physical function capacity (18). Exclusion criteria included recent myocardial infarction (1 month), recent stroke or significant cerebral neurological impairment, severe chronic obstructive pulmonary disease, and other psychiatric illnesses.
The protocol was approved by the UCSD Institutional Review Board, and participants gave written informed consent. The study was performed in accordance with the Declaration of Helsinki principles.
Depressive symptom severity
Depressive symptoms were assessed with the 21-item Beck Depression Inventory (BDI) where scores ≥ 10 indicate possible clinical depression (19). The BDI was developed to assess depressive symptoms that correspond to the Diagnostic and Statistical Manual of Mental Disorders–IV (DSM-IV) criteria for major depressive disorder (MDD) (20). A modified Structured Clinical Interview for DSM-IV (SCID) (21) was used to evaluate for MDD. Those diagnosed with MDD were referred to their treating physician, but allowed to remain in the study.
Exercise testing
Testing began at approximately 1100h. Participants abstained from physical exercise, alcohol, aspirin and caffeinated beverages the evening prior to testing and from food and drink (other than water) for two hours before testing. An intravenous (IV) catheter was placed in the antecubital vein at least one hour prior to testing and blood samples obtained prior to, and immediately post-exercise, all while the subject was in an upright, sitting posture. A blood pressure cuff was placed on the opposite arm of the IV and was connected to a Dinamap machine for automatic measurements of heart rate and systolic and diastolic blood pressure throughout the session. Subjects performed a mild graded stationary bicycle task (Viasprint 150p, Viasys, Yorba Linda, CA) consisting of a 5 minute warm-up, 10-min steady state, and 2-min cool-down. The Borg’s ratings of perceived exertion (RPE) scale (22) was used to obtain similar exercise intensity relative to existing fitness levels of all participants; the resistance (watts) was gradually increased during the warm-up period to reach the rating of 12–13 (“somewhat hard”) which was maintained for the 10-min steady state by adjusting the resistance and speed of cycling. Based on our previous studies (23), rate of perceived effort (RPE) of 12–13 consistently corresponds to 65–70% of VO2peak regardless of fitness levels.
CTX of peripheral blood mononuclear cells assay
Isoproteronol (1nM, 10nM and 100nM) was used as a chemoattractant as in previously reported methods (24). Briefly, at pre- and post- exercise time points 10 ml of blood were collected into heparinized tubes and processed within 3 hours. PBMCs were separated from whole blood with Ficoll-Hypaque and resuspended in serum free media. Cells were incubated in the dark for 45 minutes at room temperature, shaking lightly with 0.1 uM calcein-AM (acetomethyl ester)/ 2 × 106 cells per ml (25). Cells were washed and resuspended to 3 × 106 cell/ml in media with 0.1% bovine serum albumin (CTX buffer). In a modified Boyden chamber (Neuroprobe, Gaithersburg, MD) 29.5 uL/ of ISO or CTX buffer were pipetted to the bottom wells. Twenty uL of cell suspension was pipetted on a membrane above the chemoattractants and incubated for two hours at 37°C. The membrane was then submerged in PBS and non-migrated cells were scraped away with PBS dampened cotton swabs. Once dry, the membrane was read by a fluorescence plate reader (CytoFluor) at an excitation of 485 nm and emission of 530 nm.
Norepinephrine and epinephrine measures
A sub-group of 80 HF and control subjects also had blood drawn into EDTA-coated vacutainer tubes (BD Biosciences, San Jose, CA, USA) for catecholamines, NE and E. Samples were centrifuged and plasma was stored at −80°C until analysis. Plasma NE and EPI levels were determined using a COMT-based radioenzymatic assays with a preconcentration step that extracted catecholamines from 1 ml plasma and concentrates them in 0.1 ml of dilute acid following previous methods (26). The inter-assay coefficient of variation (CV) was 11% and the intra-assay coefficient of variance was 6.5%.
Statistical Analyses
Calculations were performed using SPSS Inc. (v15) software packages (SPSS, Chicago, IL). Missing data cases were excluded listwise (27). Skewed data distribution was determined by the Kolmogorov-Smirnov test and variables not normally distributed were log transformed. We controlled for the cardiovascular risk factors: age, gender, BMI and physical function (distance walked in 6 minutes) in all analyses.
Group differences in sociodemographic and medical characteristics (Table 1) were computed using independent t-tests, or for categorical data, Kruskal-Wallis tests. Repeated measures analyses of covariance (ANCOVA) were performed on CTX data with two between factors for group (HF patients and non HF controls), three within factors for dose of ISO (1, 10 and 100nM) and two within factors for time (pre- and post- exercise). The Greenhouse-Geisser correction was applied to correct for multiple comparisons. Post-hoc analyses determined baseline and exercise response differences between HF patients and non HF controls.
Table 1.
Sociodemographic and Medical Characteristics of the Study Subjects
| HF patients | controls | p | |
|---|---|---|---|
| BDI score | 11.5 ± .85 (0–34) | 5.4 ± .88 (0–44) | .002 |
| Age [years] | 59.5 ± 1.3 (30–83) | 52.2 ± 1.3 (34–84) | .001 |
| Men | 80.8 % | 47.6 % | <.001 |
| Body mass index [kg/m2] | 31.7 ± .85 (19.4–59.0) | 28.3 ± .79 (18.5–54.5) | .033 |
| Mean arterial blood pressure [mmHg] | 92.6 ± 2.2 (59.0–141.9) | 98.7 ± 1.5 (77.4–123.7) | .038 |
| Current smokers | 16.1 % | 10.6 % | .32 |
| HF severity | |||
| 6-minute walk test [meter] | 339.2 ± 10.2 (100–624) | 493. ± 13.7 (198–975) | <.001 |
| Ejection fraction [%] | 32.0 ± 1.1 (10–70) | Not measured | |
| NYHA classification II | 85.5 % | 0 % | <.001 |
| NYHA classification III | 12.7 % | 0 % | <.001 |
| Medication | |||
| ACE-blocking agents | 73 % | 0 % | <.001 |
| Beta blockers | 96.2 % | 0 % | <.001 |
| CCB | 12.1 % | 0 % | <.001 |
| Statin | 64.3 % | 0 % | <.001 |
| Aspirin | 63.3 % | 5.7 % | < .001 |
| Diuretics | 88 % | 0 % | <.001 |
| Anti-arrhythmics | 13.8 % | 0 % | <.001 |
| Digoxin | 52.5 % | 0 % | <.001 |
Data are presented as mean ± standard error of means (range) or percentage value, BDI: Beck Depression Inventory, CCB: Calcium channel blockers. Mean arterial pressure was calculated from resting BP readings (1/3 systolic BP + 2/3 diastolic BP) and body mass index (BMI) was calculated by the formula: weight in kg / (height in m)2.
Linear regression analyses determined associations between circulating catecholamine levels, heart rate and CTX to ISO 10nM (this concentration was chosen because it elicited a middle range of immune responsiveness), controlling for age, gender, BMI and physical function. We tested depressive symptom severity modulation according to Baron & Kenny (28): To test for mediation, we entered BDI as a covariate. To test for moderation, we entered the interaction between BDI and group (HF status) while controlling for group and BDI. Similarly, we entered the interaction between BDI, group and NE level (or heart rate) while controlling for BDI, group and NE (or heart rate). To graphically illustrate our findings we split BDI scores into ≥ 10, and < 10 and both heart rate and NE into “hi” and “lo” using median split (Figure 2, Figure 3 and Figure 4).
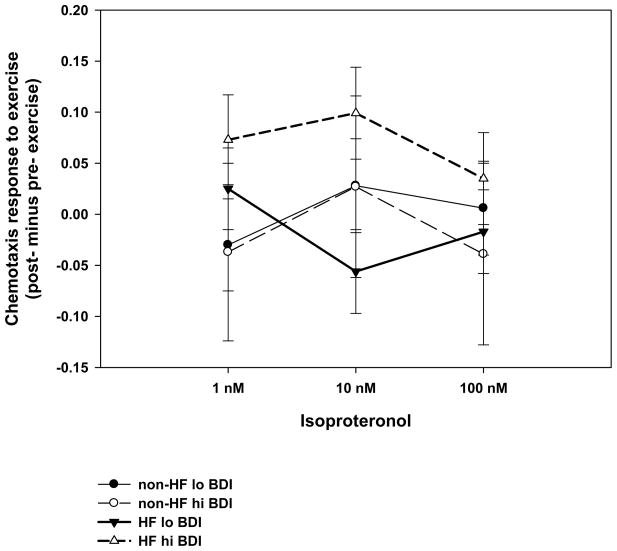
Figure 2. Chemotaxis responses to exercise in high and low depressed subjects.
The figure depicts change scores (post- minus pre- exercise) and CTX to three concentrations of isoproteronol (1nM, 10nM and 100nM) in HF patients and non-HF controls. High versus low depression are determined by scores ≥ 10 and < 10 on the Beck Depression Scale. Values are means ± SEM.
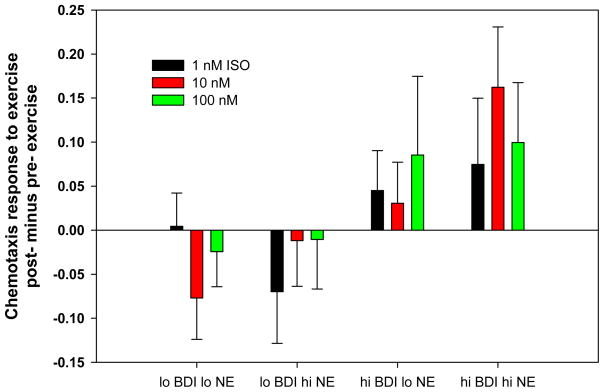
Figure 3. Chemotaxis responses to exercise with differential depression and norepinephrine levels.
The figure depicts change scores (post- minus pre- exercise) and CTX to three concentrations of isoproteronol (ISO)(1nM, 10nM and 100nM). Data from HF patients and non-HF controls were combined. High versus low depression are determined by scores ≥ 10 and < 10 on the Beck Depression Scale. High versus low Norepinephrine (NE) levels were determined by a median split. Values are means ± SEM.
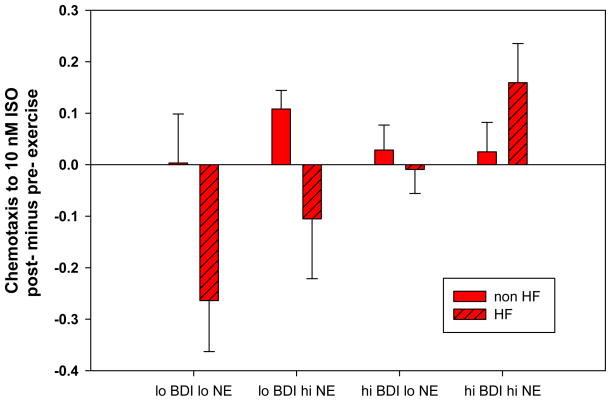
Figure 4. Chemotaxis responses to exercise in HF and controls.
The figure depicts change scores (post- minus pre- exercise) and CTX to isoproteronol (ISO) (10nM). Data from HF patients and non-HF controls are presented. High versus low depression are determined by median split on the Beck Depression Scale (≥ 7 and < 7 to depict at least 9 subjects per group). High versus low Norepinephrine (NE) levels were determined by median split. Values are means ± SEM.
RESULTS
Sociodemographic and medical characteristics of the study groups
Of the 124 subjects in that participated in the study, six were dropped from analyses because of missing data, including BDI scores (n = 2 HF subjects) and/or 6-minute walk tasks (n= 3 non HF and n = 1 HF subjects). According to Kolmogorov-Smirnov tests, BDI scores and NE were not normally distributed (p = .002 and p=.005 respectively). Standard transformation (e.g., square-root and log) did not normalize the distribution of BDI scores (p = .011) while log transformation yielded a normal distribution for NE (p=.51). However, a normal probability P-P plot suggested that log transformed BDI scores had a linear pattern with only minor deviations from the line fit to the points on the probability plot indicating that BDI scores approach a normal distribution. Table 1 presents the biological and medical characteristics of HF patients and controls. None of the control subjects and 93% of the HF patients were taking β-blockers including 67 % taking carvedilol, which has β-1, β-2 and weak alpha-1 blocking activity, 21% taking β-1 specific agents such as metoprolol. No differences were found between HF patients among β-blocking agents or not taking β-blockers for CTX-I at rest (p’s > .21) or response to exercise (p’s > .5), . Although HF patients had higher BDI scores (p < .001), three of the non-HF subjects and only one of the HF patients were diagnosed with major depressive disorder. The BDI scores (mean ± S.D.) of HF patients taking β-1 specific blockers was 7.5 ± 4.5, β-1 and β-2 blockers was 12.8 ± 8.5 and not taking β-blockers was 13.4 ± 7.1. B-blocker types or not taking β-blockers did not differ for BDI scores after controlling for NYHA class (p = .25). The lack of an association between β-blocker use and depressive symptoms is consistent with the larger literature in non-HF populations e.g. (29).
CTX-I at baseline and after exercise (Figure 1)
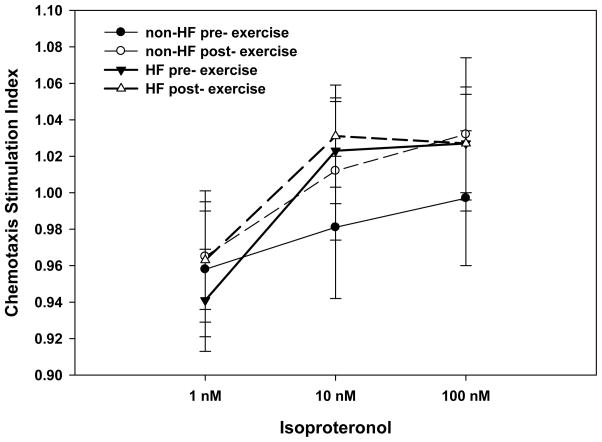
Figure 1. In Vitro chemotaxis to beta-agonist pre- and post- exercise.
The figure depicts a stimulation index (CTX to ISO/random migration) in a dose response to isoproteronol in HF patients and controls at rest and in response to exercise. Values are means ± SEM.
Repeated measures ANCOVA indicated that HF patients and non HF controls differentially responded to dose of ISO (1nM, 10nM and 100nM) while controlling for age, gender, BMI and physical function (HF status by dose interaction, F(6,112) 4.2, p = .018) after Greenhouse-Geisser correction. In order to determine the characteristics of the differences, post-hoc analyses revealed that at rest HF patients showed a positive CTX dose-response to ISO (1nM, 10nM and 100nM), while HF controls did not exhibit a CTX dose response to ISO (HF status by dose interaction at rest, p = .002). However, in response to exercise both groups had a similar positive CTX dose-response to ISO and did not differ from each other (HF status effect, p = .47).
Depressive symptoms and CTX-I pre- and post- exercise (Figure 2)
Regression analyses revealed that in both groups higher BDI scores were associated with a trend for lower CTX-I at baseline (p =.099). While in response to exercise, BDI scores appeared to moderate an increased CTX-I response in HF patients compared to non-HF controls (t = 3.3, p=.001, ΔR2 = .11).
NE, E, heart rate and CTX-I response to exercise: interaction with depression (Figures 3 and 4)
A linear regression analyses found that higher resting NE levels were associated with greater CTX-I responses to exercise in HF patients but not in non HF controls (NE by HF interaction, p = .03, ΔR2 = .045). This suggests that basal sympathetic activation may moderate CTX-I in response to exercise in HF patients. Meanwhile, baseline EPI levels and HR were not associated with CTX-I in either group (p = .50, p = .95 respectively). Furthermore, there was not an interaction between BDI and NE, E, or heart rate for CTX-I in either group.
NE, EPI and heart rate reactivity to exercise: interaction with depression
Neither NE nor E significantly increased pre- to post- exercise in either group (p = .95, p = .29 respectively), and HF patients and non-HF controls did not differ in NE or E levels in response to exercise (p = .32, p = .12 respectively). BDI scores were also not significantly associated with NE or E levels at baseline or in response to exercise.
Heart rate did not differ between HF and non HF controls at baseline (p = .22) or in response to exercise (p = .32). However, exercise produced significant increases in heart rate amongst both groups (p = .003, ΔR2 = .29). Furthermore, greater depression symptom levels were related to higher baseline heart rate, even after controlling for HF status (p = .016, ΔR2 = .045).
DISCUSSION
The current results suggest that immune cell mobility is likely differentially regulated by neuroendocrine processes in HF patients compared to non-HF controls. Moreover, elevated depression symptoms in HF patients may further augment immune cell motility to neurohormones in reaction to physical exertion. Both at rest and in response to exercise, PBMCs from HF patients exhibited a CTX dose response to ISO in vitro. This suggests that HF patients are sensitive to changes in adrenergic stimuli during both inactivity and activity. Meanwhile, non-HF controls had little response to ISO at rest, whereas in response to exercise they exhibited an increase in CTX at higher concentrations of ISO. These findings are consistent with observations that physically healthy adults have greater β-AR density and sensitivity responses to acute challenges (11), which may underlie increased homing of lymphocytes to higher concentrations of β-agonists (6,30).
Acute challenges such as exercise tasks create a window into complicated physiological processes (31) and can reveal neuroimmune dysregulation in cardiovascular disease patients that may be masked under resting conditions (13). Our principle finding was that in response to exercise HF patients with higher depression scores had greater PBMC mobility to β-adrenergic agonist (ISO), as compared to non HF controls. Meanwhile, HF patients with lower depression symptoms responded minimally to exercise, which is to be expected in a group taking β-blockers. Thus, HF patients with high depression symptoms appeared to override the effects of β-blockers. Our results are consistent with findings that psychological factors are associated with reduced β-blockade efficacy in response to exercise challenge (32).
NE, E and heart rate were assessed to explore endogenous sympathetic/neuroendocrine influences on CTX-I. Unexpectedly, NE and E levels did not increase during exercise in either HF patients or non HF controls. This is likely due to less exertion expenditure from the exercise task in the present study (approximately 65–70% of VO2peak) because of the limited exercise capacity of HF patients. In contrast various investigations of non-HF cardiovascular disease patients have used the standard Bruce protocol to obtain VO2max to examine catecholamine responses e.g. (33). Also unexpectedly, there were no differences observed between HF patients and non-HF controls for NE or EPI levels or differences between groups in exercise-induced increases in NE and EPI levels. Furthermore, heart rate was lower in HF patients than non-HF controls. These results appear in contrast to what is known about HF, being in a state of generalized sympathetic activation (34). However, medications regularly prescribed to treat HF such as βblockers likely reduced sympathetic activity in the HF patients.
Meanwhile, HF patients with elevated resting NE levels had an increase in CTX-I in response to exercise. Whereas, exercise induced changes in NE, E and heart rate were not associated with CTX-I. These findings are consistent with literature over two decades that suggest acute adrenergic exposure during exercise is not long enough to generate structural changes in lymphocyte adrenergic receptors (10). Instead, the immune cells that reside in lymphatic tissue, and that are released with physical exertion, are likely to already have altered sensitivity to βagonists. This suggests systemic neuroimmune dysregulation, which may have clinical relevance in that altered β-adrenergic receptor expression dynamics predicts development of preclinical states such as greater left ventricular mass and blood pressure (35,36).
Although we found both depression symptoms and resting NE levels were positively associated with CTX-I to exercise in HF patients, they were independent of each other. Therefore, the effects of elevated depression symptoms on CTX to βagonist were likely not directly related to heightened basal NE levels in HF patients. Instead it may again suggest neuroimmune dysregulation, since chronic psychological distress is known to be associated with down regulate β-adrenergic receptor expression (37). This in turn, would likely reduce chemoattraction sensitivity to βagonists, as seen in chronically stressed Alzheimer caregivers (25). However, HF patients with elevated depression symptoms appear to not follow this expected pattern. Thus, the mechanism that induces increased PBMC mobilization to βagonist in HF patients with depression has yet to be determined.
Limitations of the study include a disproportionate number of women in the control group compared with the HF patients in the present study. However, gender was controlled in all analyses and furthermore, results were not different when women were removed from the analyses (data not shown). Nonetheless, future studies with larger cohorts of women should be performed to explore whether there are gender differences in neuroimmune modulation in HF patients with depression. A potential limitation was the heterogeneous population, including HF patients with preserved systolic function (n = 11) and those with systolic dysfunction (n = 69). However, these groups did not differ in BDI scores (p = .20), CTX-I at baseline (p = .36) or in response to exercise (p = .79). It is important to note, that the associations found in this study are correlational and therefore may not be causative and are based upon relatively few patients. Further study is needed to replicate our findings and research is needed to tease apart the interactions between depression, sympathetic activity and HF status. In addition, research is necessary to determine if cardiac structural changes are linked with the β-adrenergic associated immune activation in this group to reveal clinical implications of these results.
In conclusion, our results suggest that HF patients with greater depression symptoms are associated with an augmented CTX-I response to physical exertion. This may indicate an increase in β-adrenergic sensitivity in HF patients with depression symptoms that override medications prescribed to reduce sympathetic activity. Furthermore, chronic sympathetic activation and depression symptoms that occur concomitantly in HF patients, could lead to even greater immune mobility in this group which may promote increased non-specific infiltration of immune cells into cardiovascular tissue and result in remodeling. Further understanding the relationship between depression symptoms and immune responses to adrenergic agonists may be useful for development of potential treatments to abrogate increased morbidity and mortality in HF patients with depression.
Table 2.
Correlation Coefficients among Variables
| Resting HR | Peak HR during EX | Av. Watts during EX | Baseline NE | Baseline EPI | Pre- to post- exercise NE | Pre- to post- exercise EPI | Pre- to post- exercise CTX (10nM ISO) | ||
|---|---|---|---|---|---|---|---|---|---|
| BDI | Non-HF | .394** | .053 | .003 | −.076 | .064 | .081 | −.139 | −.179 |
| HF | .139 | .098 | −.100 | −.006 | .007 | .080 | .087 | .238* | |
| Resting HR | Non-HF | 1.00 | .334** | −.165 | .243 | .135 | −.055 | −.129 | .093 |
| HF | .460** | −.129 | .050 | .184 | .229 | .190 | .146 | ||
| EX Peak HR | Non-HF | 1.00 | .441** | .310* | .350* | .258 | −.141 | −.135 | |
| HF | .160 | −.046 | −.050 | .417** | .208 | .045 | |||
| Av. Watts EX | Non-HF | 1.00 | −.160 | .074 | .250 | −.098 | .010 | ||
| HF | −.168 | .105 | .029 | −.001 | −.061 | ||||
| Baseline NE | Non-HF | 1.00 | .258* | −.442** | −.265 | .093 | |||
| HF | .286* | −.293* | −.222 | .320* | |||||
| Baseline EPI | Non-HF | 1.00 | .041 | −.357* | −.125 | ||||
| HF | −.151 | −.241 | .151 | ||||||
| Pre- to post-exercise NE | Non-HF | 1.00 | .457** | −.259 | |||||
| HF | .572** | −.028 | |||||||
| Pre- to post-exercise EPI | Non-HF | 1.00 | −.178 | ||||||
| HF | −.047 |
Correlation table in heart failure patients and non-heart failure controls. BDI = Beck Depression Index, HF = heart failure, HR = heart rate, EX = exercise, NE = norepinephrine, EPI = epinephrine, CTX = chemotaxis.
Statistical significant correlations of p<.05,
Significant correlations of p<.01.
Table 3.
Regression Outcomes Predicting changes in CTX to 10nM response to exercise; BDI and NE as a potential moderators
| Model | Coefficients | |||
|---|---|---|---|---|
| R2 delta | t | p | β | |
| Risk factors | .024 | |||
| Age | .487 | .628 | .001 | |
| Gender | .181 | .857 | .011 | |
| BMI | 1.361 | .178 | .005 | |
| 6-mw | .302 | .764 | .0001 | |
| HF status | −3.014 | .004 | −.985 | |
| Predictors | .146 | |||
| NE | 3.125 | .003 | .171 | |
| BDI | 3.119 | .003 | .091 | |
| Interactions | .153 | |||
| HF × NE | 2.205 | .031 | .118 | |
| HF × BDI | 3.446 | .001 | .105 | |
The final regression model and coefficients are shown. The regression models were as follows: Cardiovascular risk factors (Step 1: age, gender, BMI, 6 min walk, HF status), Predictors of CTX-I (Step 2: resting NE and BDI), and Interaction Variables (Step 3: HF status × NE and HF status × BDI). BMI = body mass index, HF = heart failure, NE = norepinephrine, BDI = Beck Depression Inventory.
Acknowledgments
Work was supported by grants HL-073355 and HL-57265 National Institutes of Health, NHLBI.
- CTX
chemotaxis
- CTX-I
chemotaxis to isoproteronol
- ISO
isoproteronol
- HF
heart failure
- PBMC
peripheral blood mononuclear cells
- NE
norepinephrine
- EPI
epinephrine
- BDI
Beck Depression Inventory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 2.Pace TW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 3.Gold SM, Zakowski SG, Valdimarsdottir HB, Bovbjerg DH. Higher Beck depression scores predict delayed epinephrine recovery after acute psychological stress independent of baseline levels of stress and mood. Biol Psychol. 2004;67:261–73. doi: 10.1016/j.biopsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Pyo RT, Sui J, Dhume A, et al. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–44. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangogiannis NG, Dewald O, Xia Y, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–92. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 6.Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000;67:553–8. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- 7.Nance D, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders VM. Interdisciplinary research: noradrenergic regulation of adaptive immunity. Brain Behav Immun. 2006;20:1–8. doi: 10.1016/j.bbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Kruger K, Lechtermann A, Fobker M, Volker K, Mooren FC. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. 2008;22:324–38. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Maisel A, Phillips C, Michel M, Ziegler M, Carter S. Regulation of cardiac beta-adrenergic receptors by captopril. Implications for congestive heart failure. Circulation. 1989;80:669–75. doi: 10.1161/01.cir.80.3.669. [DOI] [PubMed] [Google Scholar]
- 11.Redwine LS, Jenkins F, Baum A. Relation between beta-adrenergic receptor density and lymphocyte proliferation associated with acute stress. Intern J Behav Med. 1996;3:337–353. doi: 10.1207/s15327558ijbm0304_4. [DOI] [PubMed] [Google Scholar]
- 12.Redwine L, Snow S, Mills P, Irwin M. Acute psychological stress: effects on chemotaxis and cellular adhesion molecule expression. Psychosom Med. 2003;65:598–603. doi: 10.1097/01.psy.0000079377.86193.a8. [DOI] [PubMed] [Google Scholar]
- 13.Kop WJ, Weissman NJ, Zhu J, et al. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008;101:767–73. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein A, Deuster P, Francis J, Bonsall R, Tracy R, Kop W. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010 doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silberman D, Ayelli-Edgar V, Zorrilla-Zubilete M, Zieher L, Genaro A. Impaired T-cell dependent humoral response and its relationship with T lymphocyte sensitivity to stress hormones in a chronic mild stress model of depression. Brain Behav Immun. 2004;18:81–90. doi: 10.1016/s0889-1591(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 16.Redwine L, Wirtz P, Hong S, et al. A potential shift from adaptive immune activity to nonspecific inflammatory activation associated with higher depression symptoms in chronic heart failure patients. J Card Fail. 2009;15:607–15. doi: 10.1016/j.cardfail.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirtz P, Hong S, Redwine L, et al. Depressive symptoms are associated with soluble P-selectin reactivity to acute exercise in heart failure. Biol Psychiatry. 2009;65:801–7. doi: 10.1016/j.biopsych.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 18.O'Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–82. doi: 10.1136/hrt.80.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck AT. Depression inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- 20.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 22.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 23.Hong S, Johnson TA, Farag NH, et al. Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J Appl Physiol. 2005;98:1057–63. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- 24.Redwine LS, Pert CB, Rone JD, Nixon R, Vance M, Sandler B, Lumpkin MD, Dieter DJ, Ruff MR. Peptide T blocks GP120/CCR5 chemokine receptor-mediated chemotaxis. Clinical Immunol. 1999;93:124–31. doi: 10.1006/clim.1999.4771. [DOI] [PubMed] [Google Scholar]
- 25.Redwine L, Mills PJ, Sada M, Dimsdale J, Patterson T, Grant I. Differential immune cell chemotaxis responses to acute psychological stress in Alzheimer caregivers compared to non-caregiver controls. Psychosom Med. 2004;66:770–5. doi: 10.1097/01.psy.0000138118.62018.87. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 27.Allison P. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112:545–57. doi: 10.1037/0021-843X.112.4.545. [DOI] [PubMed] [Google Scholar]
- 28.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 29.Ko D, Hebert P, Coffey C, Sedrakyan A, Curtis J, Krumholz H. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288:351–7. doi: 10.1001/jama.288.3.351. [DOI] [PubMed] [Google Scholar]
- 30.Luethviksson BR, Gunnlaugsdottir B. Transforming growth factor-beta as a regulator of site-specific T-cell inflammatory response. Scand J Immunol. 2003;58:129–38. doi: 10.1046/j.1365-3083.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- 31.Linden W, Gerin W, Davidson K. Cardiovascular reactivity: status quo and a research agenda for the new millennium. Psychosom Med. 2003;65:5–8. doi: 10.1097/01.psy.0000046076.93591.ad. [DOI] [PubMed] [Google Scholar]
- 32.Rutledge T, Linden W, Davies RF. Psychological risk factors may moderate pharmacological treatment effects among ischemic heart disease patients. Canadian Amlodipine/Atenolol in Silent Ischemia Study (CASIS) Investigators. Psychosom Med. 1999;61:834–41. doi: 10.1097/00006842-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Hughes JW, York KM, Li Q, Freedland KE, Carney RM, Sheps DS. Depressive symptoms predict heart rate recovery after exercise treadmill testing in patients with coronary artery disease: results from the Psychophysiological Investigation of Myocardial Ischemia study. Psychosom Med. 2008;70:456–60. doi: 10.1097/PSY.0b013e31816fcab3. [DOI] [PubMed] [Google Scholar]
- 34.Floras J. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Choi D, Kim C. Hypertensive left ventricular hypertrophy: relation to beta-adrenergic receptor kinase-1 (betaARK1) in peripheral lymphocytes. J Hypertens. 2004;22:1025–32. doi: 10.1097/00004872-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Treiber F, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Dimsdale JE, Mills P, Patterson T, Ziegler M, Dillon E. Effects of chronic stress on beta-adrenergic receptors in the homeless. Psychosom Med. 1994;56:290–5. doi: 10.1097/00006842-199407000-00003. [DOI] [PubMed] [Google Scholar]