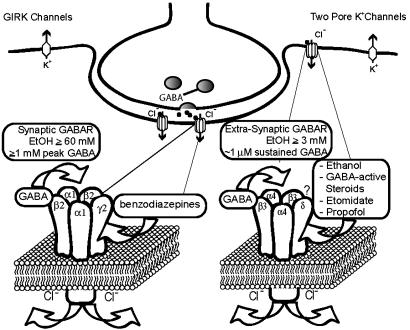
Fig. 4.
Synaptic versus extrasynaptic receptors. Synaptic receptors (shown is the most prevalent α1β2γ2 synaptic GABARs) respond to saturating GABA (>1 mM peak GABA concentrations) and show high efficacy but fairly low potency (45). In contrast, extrasynaptic receptors (composed of α4δ- or α6δ- and most likely β3-subunits) are activated by persistent and usually nonsaturating ambient GABA concentrations (0.5-1 μM), and, even at saturating GABA concentrations, are characterized by low-current levels (high-potency, low-efficacy receptors). We suggest a model where EtOH and other anesthetics lead to an increase in GABA efficacy (increase in open probability and/or possibly single-channel conductance), which leads to increased Cl- current. A massive increase in GABA-activated Cl- conductance by anesthetics could completely silence neurons expressing δ-subunit-containing GABARs, thereby producing anesthesia. The activation of extrasynaptic GABARs is functionally equivalent to activation of background K+ channels. G protein-coupled inwardly rectifying K+ (GIRK) channels have been shown to respond to fairly low concentrations of EtOH (3, 4) and may mediate EtOH analgesic actions (5). GIRK channels may also contribute to the anesthetic actions of volatile anesthetics (48, 49). The two-pore K+ channels, TASK1 and TREK, are likely targets for volatile anesthetics (35, 36, 50, 51). The functional similarity between extrasynaptic GABARs and two-pore K+ channels is supported by the finding that, in cerebellar granule cells, a background potassium channel (most likely TASK1), compensates for the loss of extrasynaptic GABARs in mice lacking the GABAR α6-subunit (37).