Abstract
A gene vaccine based on a mammalian expression vector containing the sequence of a peptide mimotope of Phl p 5 was constructed. To test whether mimotope gene vaccines can induce allergen-specific antibody responses via molecular mimicry, BALB/c mice were immunized using the mimotope construct with or without a tetanus toxin T-helper epitope. Moreover, intradermal injection was compared to epidermal application via gene gun immunization.
Immunization with both mimotope gene constructs elicited allergen-specific antibody responses. As expected, gene gun bombardment induced a Th2-biased immune response, typically associated with IgG1 and IgE antibody production. In contrast, intradermal injection of the vaccine triggered IgG2a antibody expression without any detectable IgE levels, thus biasing the immune response towards Th1. In an RBL assay, mimotope-specific IgG antibodies were able to prevent cross-linking of allergen-specific IgE by Phl p 5. A construct coding for the complete Phl p 5 induced T-cell activation, IFN-γ and IL-4 production. In contrast, the mimotope-DNA construct being devoid of allergen-specific T-cell epitopes had no capacity to activate allergen-specific T cells.
Taken together, our data show that it is feasible to induce blocking IgG antibodies with a mimotope-DNA construct when applied intradermally. Thus the mimotope-DNA strategy has two advantages: (1) the avoidance of IgE induction and (2) the avoidance of triggering allergen-specific T-lymphocytes. We therefore suggest that mimotope gene vaccines are potential candidates for epitope-specific immunotherapy of type I allergy.
Keywords: Allergy, DNA vaccine, Mimotope
1. Introduction
Phage display libraries can be screened with allergen-specific IgE antibodies to identify mimics of allergenic B-cell epitopes. The selected mimics resemble IgE binding epitopes of the allergen surface by virtue of their physico-chemical properties [1]. In peptide form, such epitope-mimics (mimotopes) have repeatedly been shown to induce epitope-specific, allergen-specific immune responses [2–6]. Due to the fact that mimotopes do not contain allergen-specific T-cell epitopes, the immune responses towards mimotopes depend on the recruitment of bystander Th-cells. In a previous study we could show that mimotopes per se were not cross-reactive with the allergen at the T-cell level and that only phage-displayed mimotopes could activate T-cells to generate a mimotope-specific antibody response [5]. This unspecific bystander T-cell help can be provided by the phage coat protein pIII, which is composed of 406 amino acids [7]. The mimotope-induced IgG antibodies are then directed not only against the mimotopes, but co-recognize the 3-dimensional allergen epitope via molecular mimicry. Therefore, they are able to prevent the high-affinity interaction between allergen and specific IgE antibodies [3,8] and thus can be called blocking antibodies [9,10]. In several studies we and others have already characterized peptides and anti-idiotypic Fab fragments being mimotopes of different allergens and antigens[2,6,11–13].
Besides attempts to improve protein-based allergen immunotherapy by generation of allergen mutants, hypoallergens or peptides, immunization experiments with allergen-encoding DNA have yielded promising results. DNA vaccination does not only prevent allergic sensitization, but is also capable to modulate already ongoing Th2 responses [14–17]. Genetic immunization approaches with mimotope genes have hitherto been restricted to tumor antigens [18]. Therefore, we investigated in the present study whether this strategy could also be useful in the context of allergy therapy. For this purpose, we designed the construct “pCMV-F1”, a gene vaccine composed of a mimotope of the grass pollen allergen Phl p 5 and phage coat protein pIII, the latter serving as (i) a “non-allergenic” carrier protein, (ii) a source of T-helper epitopes and (iii) a stabilizer of the three-dimensional exposure of the mimotope. In a complementary strategy to further enhance the immunogenicity of the mimotope construct, a promiscuous tetanus toxin T-helper epitope from Clostridium tetani [19] was additionally introduced into a second construct, designated pCMV-F1/Tet.
As the route of the DNA application might critically affect the cytokine milieu and thus the outcome of immunizations [20],we aimed to compare head to head two different routes of gene vaccine administration. Whereas gene gun immunization using only minute amounts of DNA has been demonstrated to trigger a pre-dominant Th2 type immune response, intradermal application is known to recruit efficiently Th1 cells, possibly also due to the delivery of higher amounts of DNA [21,22].
2. Materials and methods
2.1. Construction of the DNA immunization vectors pCMV-F1 and pCMV-F1/Tet
In a previous study, peptide mimotope F1 was identified as a specific epitope-mimic (mimotope) of grass pollen major allergen Phl p 5 by screening a phage display library with Phl p 5-specific IgE [3]. The peptide library consisted of decameric peptides presented on minor phage coat protein pIII of filamentous phage M13. Clone F1 (SRLGRSSAWV), showing the highest binding capacity to the antibodies, was chosen for expression in the high copy number plasmid pCI (Promega, Madison, WS, gb:CVU471199). The expression cassette in pCI contains the CMV immediate-early promotor and can provide three independent multiple cloning sites for the individual in-frame cloning of, e.g. an ER-targeting leader sequence, the gene of interest and a heterologous helper epitope [23]. For cloning of pCMV-F1, a PCR reaction using the filamentous phage DNA (M13) plus mimotope insert (F1) as a template was performed. To amplify the sequence of the pIII phage coat protein together with the mimotope sequence, the following primers were used, with F1-M13 DNA serving as a template: upstream primer pIII fwd1 EcoRI 5′-CACCGAATTCATGGCTGAATGCAGTCGTCT-3′ and reverse primer pIII rv1 XbaI 5′-GGGGTCTAGATTACTCATTTTCAGGGATAG-3′. The resulting 272 bp (base pair) fragment was digested with EcoRI–XbaI and ligated into the pCMV vector being digested with the same restriction enzymes.
For the construction of the pCMV-F1/Tet vector (see Fig. 1), inclusion of a foreign 63 bp long toxin helper epitope (fnnftvs-fwlrvpkvsashle), here named “Tet” from C. tetani was performed [19]. A PCR reaction using the filamentous phage DNA (M13) as a template was performed (upstream primer pIII fwd1 EcoRI 5′-CACCGAATTCATGGCTGAATGCAGTCGTCT-3′ and the reverse primer pIII rv2 MluI 5′-GGGGACGCGTCTCATTTTCAGGGATAGCAA-3′). The resulting construct was digested with EcoRI–MluI and ligated into a pCMV TPA Tet vector (constructed from pCI, see [23]) digested with the same enzymes. For control, pCMV-mock, representing the vector without phage coat protein pIII and insert, and pCMV-P5 encoding the recombinant Phl p 5a (rPhl p 5), were used [24]. Successful cloning was controlled by sequencing all constructs in an Abi PrismTM Genetic Analyzer (MWG Biotech, Germany).
Fig. 1.

Map of the eukaryotic expression vector pCI. The universal expression vector contains among other features a CMV immediate early promoter-enhancer region, an optimized chimeric intron, a multiple cloning site (MCS) and the SV40 late polyadenylation site. The inset shown here comprises the phage coat protein pIII, the mimotope F1 and an additional tetanus toxin helper epitope (pCMV-F1/Tet).
The plasmids were propagated in E. coli strain XL-1 Blue and large-scale purified with an Endo Free Plasmid Giga Kit (MWG Biotech, Germany). Plasmid DNA was then analyzed for size by agarose gel electrophoresis and quantified by spectrophotometry (OD260 nm/OD280 nm). Vectors were stored in endotoxin-free water at −20 °C.
2.2. Animals
BALB/c mice (female; 6–10 weeks) were purchased from Charles River Laboratories (Sulzfeld, Germany GmbH) and treated according to European Community rules of animal care [25] with the permission of the Austrian Ministry of Science (BMBWK-66.012/0015-BrGT/2005 and BMWF-66.012/0015-CIGT/2007).
2.3. Mice and immunizations
The detailed immunization schemes are depicted in Fig. 2. Mice (n = 6) were immunized either by particle bombardment using a Helios gene gun (Bio-Rad, Munich, Germany) or by intradermal (i.d.) needle injection with pCMV-F1, pCMV-F1/Tet, pCMV-mock or pCMV-P5 into the shaved abdomen. For gene gun immunizations, plasmids were coated onto 1.6 μm gold particles and a helium discharge pressure of 400 psi was used to deliver a total of 4 μg per immunization by two shots at two ventral sites. Particle bombardment was performed on days 2, 16, 30 and finally four weeks later at day 59 according to Scheiblhofer et al. [20]. Blood samples were drawn on days 0, 15, 29, 44 and 73.
Fig. 2.
Schemes of the immunization protocols. The axis indicates 14 days intervals. Gene gun (a) and intradermal immunization protocol (b) using the constructs pCMV-F1, pCMV-F1/Tet, PCMV-mock and pCMV-P5. Subcutaneous application protocol (c) using rPhl p 5 as the immunization substance.
i.d. immunizations were performed using a slightly different protocol, according to Hartl et al. [23] with 100 μg plasmid in a volume of 200 μl PBS on days 2, 10, and 16 into 4–6 sites at the shaved abdomen. Blood samples were taken on days 0, 9, 15 and 22. According to an established immunization protocol [16], a control group of mice was immunized subcutaneously (s.c.) with 5 μg rPhl p 5 adsorbed to 100 μl Al(OH)3 in a total volume of 150 μl sterile endotoxin-free PBS.
2.4. Serum immunoglobulin detection in ELISA
For the measurement of Phl p 5-specific IgG, 96-well high-bind, flat-bottomed immunoplates (FluoroNunc, Roskilde, Denmark) were coated overnight at 4 °C with rPhl p 5 (0.1 μg per well/100 μl PBS). Plates were washed with PBS/0.1% Tween 20 and blocked for 1 h at room temperature with 200 μl/well PTB (PBS/0.1% Tween-20/0.5% bovine serum albumin (BSA)).
Sera were added at a dilution of 1:100 in 100 μl PTB for total IgG, IgG1 and IgG2a and incubated for 1 h at RT. Plates were washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (BioRad, Germany), HRP-conjugated rabbit anti-mouse IgG1 (Zymed, San Francisco, USA) or HRP-conjugated rabbit anti-mouse IgG2a (Zymed), each diluted 1:1000 in PBT for 1 h at RT.
The reaction was detected by adding Luminol (Boehringer-Mannheim, Germany) 1:2 in aqua bidest. Chemiluminescence was measured at 405–490 nm by a LucyII ELISA-plate luminometer (Anthos Labtec, Salzburg, Austria) and fluorescence was calculated in photon counts × 1000 (kilo photon counts, KPC).
2.5. β-Hexosaminidase release from rat basophil leukemia cells
RBL-2H3 cells were plated into 96-well, flat-bottomed tissue culture plates (Becton-Dickinson, Franklin Lakes, NJ, USA) at a density of 4 × 104 cells/well in 100 μl of RPMI 1640 supplemented with 10% (v/v) heat-inactivated fetal calf serum, 100 U penicillin and streptomycin/ml, 4 mM l-glutamine, 2 mM sodium pyruvate, 10 mM HEPES and 100 μM of 2-mercaptoethanol. Plates were incubated o.n. at 37 °C, 95% relative humidity, 7.5% CO2.
Subsequently, 50 μl of supernatant was removed. RBL cells were passively sensitized by adding pooled sera from either DNA-s.c. rPhl p 5-immunized mice or preimmune sera in a dilution of 1:100 for 2h at 37 °C.
RBL cells were washed twice with 200 μl/well Tyrode’s buffer [23]. Cross-linking of FcεRI-bound IgE was induced by addition of rPhl p 5 (0.3 μg/ml in Tyrode’s buffer). For maximal release, 10 μl 10% Triton X-100 was added. Sera from untreated mice served as negative control. Cells were incubated for 30 min at conditions indicated above. Thereafter, plates were centrifuged for 5 min at 1200 rpm at RT, 50 μl of supernatants were transferred to fresh plates and incubated with 50 μl 4-methyl-umbelliferyl-N-acetyl-β-d-glucosaminide (4-MUG) (Sigma, Deisenhofen, Germany) in citrate buffer (0.1 M, pH 4.5) for 1 h at indicated conditions. The reaction was stopped by adding 100 μl glycine buffer (0.2 M glycine and 0.2 M NaCl, pH 10.7) and fluorescence was measured at 465–360 nm using a microplate reader (Spectrafluor, Tecan, Austria). Results were calculated in relation to the percentage of total β-hexosaminidase released after addition of Triton X-100 (100%). The release value obtained by Triton treatment was set 100% and the other results compared hereto.
For the inhibition assay, RBL cells were passively sensitized with sera of s.c. rPhl p 5-immunized mice in a dilution of 1:100 for 2 h. Sera of mice to be tested for presence of blocking IgG antibodies were pooled and heated at 56 °C for 1.5 h to destroy the β-hexosaminidase being present in the serum. Subsequently, these sera were incubated with 0.005 μg rPhl p 5 in dilutions 0, 1:50 and 1:20 for 2 h prior to incubation with RBL cells.
2.6. Isolation of splenocytes and detection of cytokines
Mice were sacrificed and spleens were aseptically removed. Spleens were minced in 1 ml minimal essential culture medium (MEM) and aggregated cells were sedimented for 10 min. Lysis of erythrocytes was performed with 5 ml ACK (ammonium chloride lysis buffer): (150 mM NH4Cl, 10 mM KHCO3, 0.1m MNa2 EDTA dissolved in aqua bidest., pH 7.2–7.4) for 5 min at RT. Cells were resuspended in 7 ml MEM and centrifuged for 5 min at 300 × g. After repetition of the last step, cells were plated into 96-well, flat bottom tissue culture plates (BD-Falcon, NJ, USA) (2 × 105 cells/well). For stimulation, rPhl p 5 (20 μg/ml), ovalbumin (20 μg/ml) or medium were added for 48 h at 37 °C, 95% relative humidity, and 7% CO2. IFN-γ and IL-4 measurements of supernatants were performed by a Fluorescent Bead Immunoassay (Bender MedSystems, Vienna, Austria) of pure spleen supernatants according to the manufacturer’s instructions.
2.7. Detection of cytokine producing cells by ELISPOT
Anti-IFN-γ (4 μg/ml) coated ELISPOT plates (Millipore, Bedford, MA, USA) were washed with PBS and blocked with PBS/2% dry milk powder (DMP) for 2 h at RT.
At the day of sacrifice, spleens were harvested and cells were cultured at a density of 2 × 105 cells/well with rPhl p 5 (20 μg/ml) at 37 °C, 7% CO2 o.n. Plates were washed with PBS/0.1% Tween-20.
Biotinylated anti-IFN-γ antibodies (2 μg/ml in PBS/1% BSA) were added and the plates were incubated at RT for 2.5 h. Plates were washed again and incubated with streptavidin-PE (1:1000 I PBS/1% BSA) for 2 h at RT. Cytokine-producing cells per 2 × 105 seeded cells per well were detected by adding 3-amino-9-ethylcarbazole (AEC) substrate according to the manufacturer’s instructions.
2.8. Statistical analysis
Statistical comparison between groups was performed by the Mann Whitney-U test, using the software SPSS (version 14.0 for Windows). Differences were considered statistically significant at P-values < 0.05.
3. Results
3.1. Mimotope-DNA induces allergen-specific immune responses in i.d. and gene gun immunized BALB/c mice
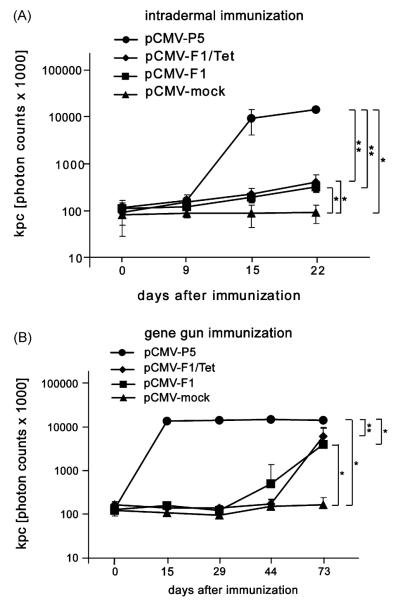
To prove the mimicry potential of the mimotope constructs pCMV-F1 and pCMV-F1/Tet, mice were immunized—either intradermally (i.d.) or by biolistic gene transfer. Sera of mice immunized with the mock vector, i.e. a construct lacking the mimotope as well as the Tet gene (pCMV-mock), served as negative control. Intradermal immunization with the gene of the entire Phl p 5-allergen (pCMV-P5) elicited a significant increase of the allergen-specific total IgG response already after two injections, as observed from sera of day 15 (P < 0.01). Three injections with both mimotope constructs were needed to induce a significant antibody response (P < 0.05), as analyzed in sera from day 22, although with 100-fold lower titres than with the allergen-encoding construct. Coexpression of the tetanus toxin helper epitope did not increase the immunogenicity of the mimotope DNA vaccine (Fig. 3A).
Fig. 3.
Phl p 5-specific IgG antibody responses after i.d. and gene gun immunization. (A) i.d. immunization of DNA mimotope constructs pCMV-F1 and pCMV-F1/Tet induced significantly higher Phl p 5-specific IgG responses after the third immunization (day 22) compared to injection of negative control pCMV-mock. Immunization with pCMV-P5 served as positive control. (B) Gene gun immunization with both DNA mimotope constructs after the fourth immunization (day 73) revealed 10-fold higher IgG levels than i.d. administration.The induction of IgG was highly specific in comparison to treatment with pCMV-mock. *P < 0.05, **P < 0.01.
As depicted in Fig. 3B, four gene gun immunizations with the mimotope constructs rendered a tenfold higher titre of Phl p 5-specific IgG antibodies than i.d. treatment. However, a single shot of the Phl p 5 DNA via gene gun (serum from day 15) was already sufficient to reach a significantly elevated level of allergen-specific IgG compared to all other groups.
3.2. Intradermal injection or epidermal gene gun application differently polarize T-helper cell responses against the mimotopes
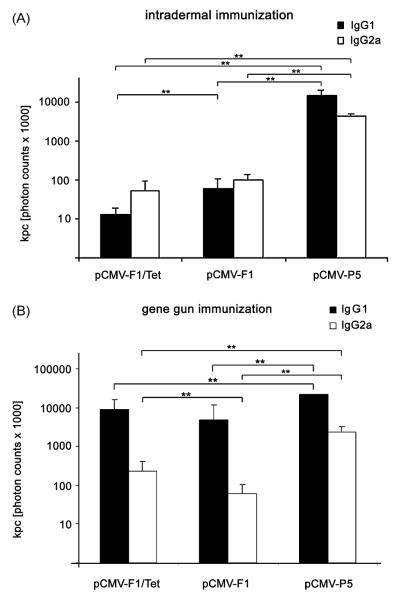
The induced antibody response was analyzed in more detail with regard to IgG1 and IgG2a levels (Fig. 4). Intradermal immunization with the vector encoding the entire gene of Phl p 5, pCMV-P5 was most effective in inducing Phl p 5-specific IgG1 and IgG2a (Fig. 4A) as seen in sera taken at day 22. The mimotope-DNA constructs pCMV-F1 and pCMV-F1/Tet induced comparable levels of anti-Phl p 5 IgG1 and IgG2a (Fig. 4A) with the IgG1 titre being lower in the pCMV-F1/Tet treated mice.
Fig. 4.
Phl p 5-specific IgG1 and IgG2a antibody subclass responses after vaccinations. (A) Sera of mice from day 22 after three intradermal immunizations with all three constructs (pCMV-F1/Tet, pCMV-F1, or pCMV-P5) rendered similar tires of IgG1 and IgG2a antibodies. (B) Mice receiving four times particle bombardment by gene gun showed higher IgG1 levels in all three DNA immunized groups (B). **P < 0.01.
Four gene gun immunizations with all three constructs induced significantly higher IgG1 compared to IgG2a levels indicating a bias towards a Th2 response (Fig. 4B). Mice immunized via gene gun with pCMV-F1/Tet rendered significantly higher IgG2a levels compared to pCMV-F1.
3.3. Gene gun but not i.d. immunization with mimotope genes triggers production of functional IgE
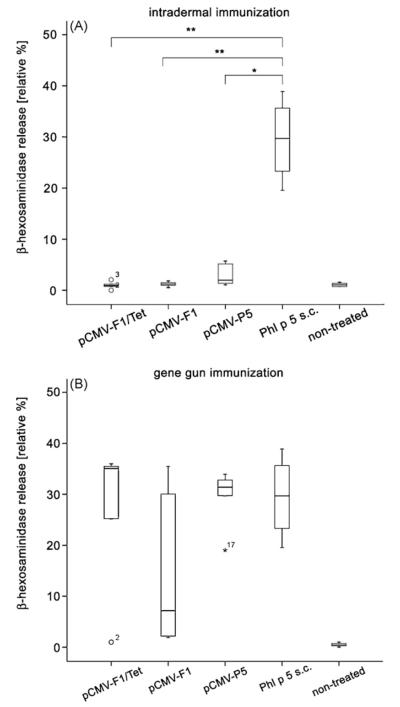
To investigate whether genetic immunization with mimotopes induces IgE antibodies which can be cross-linked by the recombinant allergen, a rat basophilic leukemia cell release assay was performed. RBL-2H3 cells were passively sensitized with sera diluted 1:100 from mice immunized either i.d. or by particle bombardment. Subsequent cross-linking was performed with rPhl p 5. IgE-containing sera of mice immunized s.c. with rPhl p 5 served as positive control [24], whereas. i.d. injection of both mimotope gene vaccines or the entire gene of Phl p 5 did not induce detectable IgE antibody levels (Fig. 5A). In contrast, gene gun immunization induced functional, although variable IgE antibody levels in all groups (Fig. 5B).
Fig. 5.
Functional IgE antibodies measured by RBL cell mediator release assay. (A) rPhl p 5 protein, but none of the mimotope-DNA constructs pCMV-F1 and pCMV-F1/Tet induced significant Phl p 5-specific IgE synthesis after i.d. immunization. (B) In contrast, gene gun injection triggered IgE induced β-hexosaminidase release in all experimental groups. Results are presented as relative percentage of total β-hexosaminidase released after addition of 10% Triton X-100. Samples showing more than 3-fold deviation from the end of the box were defined as extremes and marked with numbers and circles. Numbers represent sample numbers in the order given by the statistical program used. *P < 0.05, **P < 0.01.
3.4. Allergen mimotope gene vaccines do not stimulate allergen-specific T-cells
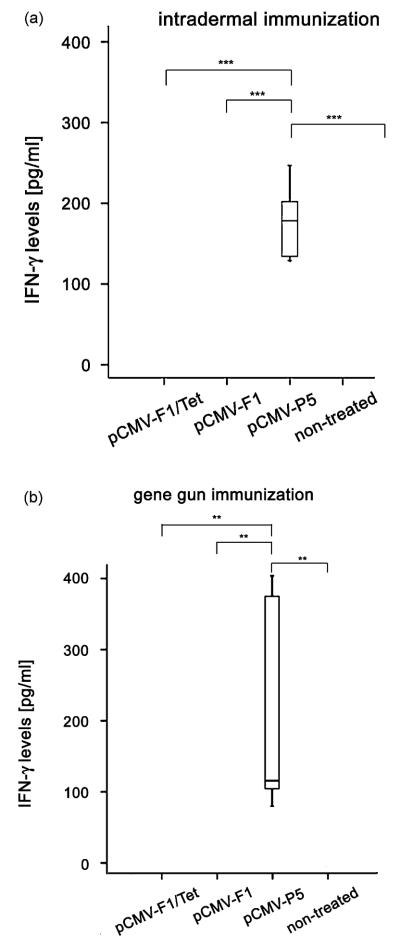
The capacity of the DNA constructs to stimulate allergen-specific T-cells was tested by measuring the production of IFN-γ and IL-4 from splenocytes of immunized mice. To determine the number of IFN-γ producing spleen cells of the animals, an ELISPOT assay was carried out. Neither i.d. nor gene gun immunization with the mimotope constructs resulted in T-cell stimulation and IFN-γ release upon allergen stimulation. Significantly increased numbers of IFN-γ-producing cells (Fig. 6A and B), and increased levels of IFN-γ in the supernatants of stimulated splenocytes (Fig. 7A and B) were only detected in mice immunized with the construct encoding the entire Phl p 5, which contained all relevant T-cell epitopes of the allergen (P < 0.001 to the i.d. group, P = 0.002 to the gene gun group).
Fig. 6.
Number of IFN-γ producing cells. Spleen cells of immunized mice were cultured on ELISPOT plates coated with anti-IFN-γ antibodies and stimulated with rPhl p 5. IFN-γ secreting cells could not be detected in the mimotope groups pCMV-F1 and pCMV-F1/Tet, neither after i.d. (A) nor after gene gun immunization (B). Only i.d. immunization with the construct encoding the entire gene pCMV-P5 resulted in IFN-γ production upon rPhl p 5-stimulation. In the gene gun treated group, only a few IFN-γ expressing cells were detected, probably due to the Th2-biased immune response after the immunization. Samples with signals showing more than 1.5-fold interquartile range deviation from the end of the box were defined as outliers and marked as circles. Numbers represent sample numbers in the order given by the statistical program used.
Fig. 7.
IFN-γ levels in supernatants of stimulated spleen cells. Spleen cells of immunized mice were stimulated with rPhl p 5 for 48h. IFN-γ secretion was only observed in spleen cell supernatants from the group immunized with the gene vaccine encoding the allergen (pCMV-P5) after i.d. (A) and gene gun immunization (B). **P < 0.01, ***P < 0.001.
IL-4 production could also be detected in mice immunized with the DNA encoding the recombinant allergen Phl p 5, treated by gene gun bombardment. Levels of all other groups were below the detection limit (Fig. 8).
Fig. 8.
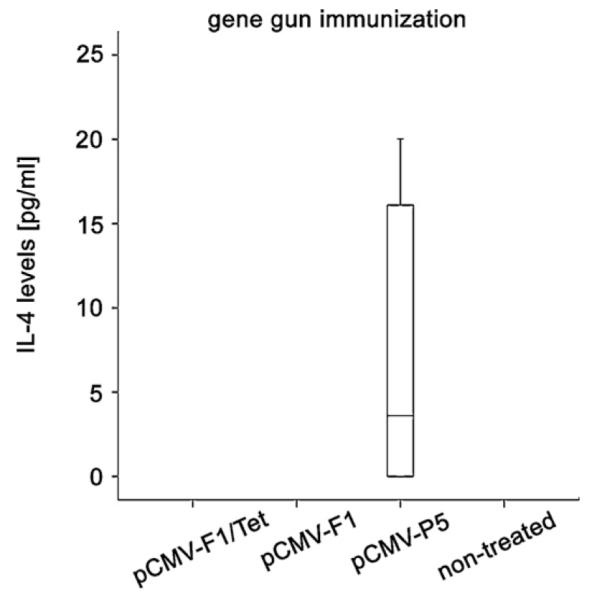
IL-4 levels in supernatants of stimulated spleen cells. Spleen cells of immunized mice were stimulated with rPhl p 5 for 48 h. IL-4 secretion was only induced in the group immunized with the gene vaccine encoding the allergen (pCMV-P5) after gene gun immunization. No IL-4 production was observed after intradermal immunization with all of the constructs.
3.5. Antibodies induced by allergen mimotope DNA act as blocking antibodies
Due to their ability to bind allergen in a specific manner (as demonstrated in Fig. 3), the anti-mimotope antibodies should be able to block the binding of allergen to allergen-specific IgE on mast cells or basophils. This was tested in an RBL-inhibition assay using pooled sera from mice immunized intradermally with mimotope gene vaccine pCMV-F1, a gene vaccine encoding the recombinant allergen Phl p 5 (pCMV-P5), or preimmune sera. The results in Fig. 9 show that preincubation of allergen with pooled sera of mice immunized with Phl p 5 DNA or mimotope-DNA significantly inhibited (P < 0.01 and P < 0.05, respectively) the IgE cross-linking capability of the allergen.
Fig. 9.
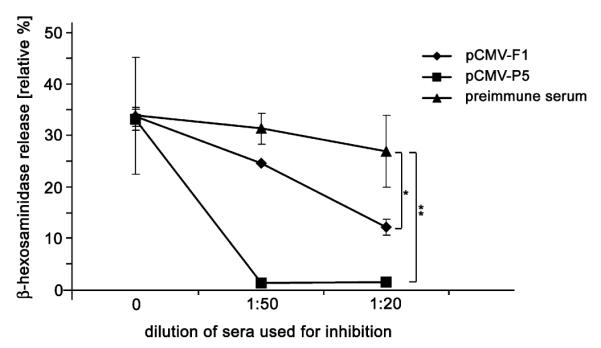
Allergen-specific blocking antibodies. Antibodies with the potential to bind rPhl p 5 and thus inhibit the cross-linking of sensitized mast cells were measured by a β-hexosaminidase release inhibition assay. Cell release was significantly reduced by preincubation of rPhl p 5 with anti-mimotope (raised i.d. with pCMV-F1) or anti-Phl p 5 sera (raised i.d. with pCMV-P5) diluted 1:20 before addition to the sensitized RBL cells. *P < 0.05, **P < 0.01.
4. Discussion
Previously, we were able to define decameric peptides as IgE-epitope mimics (mimotopes) of grass pollen allergen Phl p 5. In these studies, the potential of protein-based mimotopes for allergy immunotherapy was demonstrated by the induction of anti-mimotope antibodies in BALB/c mice which were co-reactive with Phl p 5 [3].
The similarity between mimotopes and B-cell epitopes concerns the three-dimensional structure and the distribution of the electrostatic charges, but mimotopes do rarely share sequential homologies with the antigen they mimic [26]. In the context of allergy treatment, this feature might be interesting, as a mimotope vaccine could promote the stimulation and expansion of pre-existing allergen-specific B-cells, but simultaneously prevent activation of allergen-specific T-cells. This might be advantageous as it is known that allergen-specific T-cells may act proinflammatory in late phase reactions [27]. Indeed, in the present study the in vitro analysis of Th1 associated IFN-γ production and Th2 associated cytokine IL-4 from murine spleens confirmed this hypothesis, because immunization with mimotope gene vaccines did neither stimulate proliferation nor cytokine production of allergen-specific T-lymphocytes.
The safety concerns with respect to possible anaphylactic risks for protein-based immunotherapies of allergy are an issue that might be addressed by applying mimotopes. Mimotopes induce IgG antibodies which are B-cell epitope-specific [5], thereby avoiding sensitization against neo-epitopes and an amplification of the IgE response during immunization. Moreover, the inhibition of the IgE allergen interaction by virtue of their blocking capacity has been shown [8,28]. On the other hand the potency of DNA vaccines to counteract the IgE class switch by inducing IFN-γ producing CD4+ or CD8+ T-cells has yielded promising results in the field of allergy [15]. Consequently, we attempted here to combine the specific features of genetic immunization with the beneficial effects of mimotopes.
Gene vaccines have been introduced into animal tissue by several different routes, the most abundant being intradermal, intramuscular or biolistic gene transfer. The latter has been suggested to induce a Th1 milieu with 100–1000 times less amount of DNA than intramuscular injection [29]. However, gene gun bombardment was questioned for allergen immunotherapy by several studies [22,30–33].
The aim of this study was to incorporate the sequence of a previously identified peptide mimotope of Phl p 5 into a eukaryotic expression vector, the whole construct being designated pCMV-F1. The introduced minigene encoding the mimotope peptide was fused to the pIII phage molecule relevant for the display of mimotopes during their selection. This strategy intended to stabilize the three-dimensional structure of the mimotope exactly in the way it was selected during the biopanning procedure in context with phage protein pIII [34]. PIII could further act as a carrier protein supporting T-cell bystander help, which has been postulated already in the 1980s [35]. As the mimotope per se is composed of only 10 amino acids and has been shown so far only to induce immune responses when coupled to a carrier [5], further T-cell help had to be provided. The results actually demonstrated that the size of the mimotope-pIII construct being 416 amino acids was sufficient to induce an immune response, which could not be further enhanced by the addition of a promiscuous T-cell epitope from tetanus toxin. Intradermal but not gene gun immunization with mimotope-DNA induced blocking IgG antibodies without triggering IgE production, which was demonstrated in a functional test, the RBL-assay. Gene gun-mediated transfer delivers DNA more efficiently into cells by depositing DNA coated beads within the cytoplasm, thereby leading to a higher transfection rate than does direct needle injection [36]. How this may impact IgE synthesis, remains to be answered. Our data confirm results questioning the applicability of gene gun bombardment for allergen immunotherapy by several studies [20–22]. Thus, intradermal injection only eliciting IgG1 and IgG2a, but no IgE antibodies, is the preferred route also for the mimotope gene vaccine. The induced IgG antibodies also proved to have blocking activity by preventing allergen mediated cross-linking of IgE bound via FcεRI to effector cells. The type of the immune response induced by different application routes thus gives an important hint for future applications, e.g. administration of mimotope-DNA in therapeutic settings.
From our data we conclude, that the mimotope gene-vaccine has mimicry potential of a major IgE epitope of the grass pollen allergen Phl p 5. More importantly, the Th1-biased intradermal application of the mimotope-DNA induced an efficient immune response comprising blocking IgG antibodies, simultaneously avoiding IgE expansion and stimulation of allergen-specific T-cells. Based on our data we suggest that the mimotope technology in context with gene vaccination represents a promising novel option for epitope-specific allergen immunotherapy.
Acknowledgements
This study was supported by grants of the Austrian Science Fund: SFB F1808-B13 (FWF), NFN projects S8811, S8813 and a Hertha Firnberg stipendium.
References
- [1].Pali-Scholl I, Jensen-Jarolim E. Biopanning for the characterization of allergen mimotopes. Methods Mol Med. 2008;138:271–83. doi: 10.1007/978-1-59745-366-0_23. [DOI] [PubMed] [Google Scholar]
- [2].Ganglberger E, Grunberger K, Wiedermann U, Vermes M, Sponer B, Breiteneder H, et al. IgE mimotopes of birch pollen allergen Bet v 1 induce blocking IgG in mice. Int Arch Allergy Immunol. 2001;124:395–7. doi: 10.1159/000053768. [DOI] [PubMed] [Google Scholar]
- [3].Hantusch B, Krieger S, Untersmayr E, Scholl I, Knittelfelder R, Flicker S, et al. Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol. 2004;114:1294–300. doi: 10.1016/j.jaci.2004.06.048. [DOI] [PubMed] [Google Scholar]
- [4].Suphioglu C, Schappi G, Kenrick J, Levy D, Davies JM, O’Hehir RE. A novel grass pollen allergen mimotope identified by phage display peptide library inhibits allergen–human IgE antibody interaction. FEBS Lett. 2001;502:46–52. doi: 10.1016/s0014-5793(01)02661-8. [DOI] [PubMed] [Google Scholar]
- [5].Scholl I, Wiedermann U, Forster-Waldl E, Ganglberger E, Baier K, Boltz-Nitulescu G, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy. 2002;32:1583–8. doi: 10.1046/j.1365-2222.2002.01527.x. [DOI] [PubMed] [Google Scholar]
- [6].Szalai K, Fuhrmann J, Pavkov T, Scheidl M, Wallmann J, Bramswig KH, et al. Mimotopes identify conformational B-cell epitopes on the two major house dust mite allergens Der p 1 and Der p 2. Mol Immunol. 2008;45:1308–17. doi: 10.1016/j.molimm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- [7].Kretzschmar T, Geiser M. Evaluation of antibodies fused to minor coat protein III and major coat protein VIII of bacteriophage M13. Gene. 1995;155:61–5. doi: 10.1016/0378-1119(94)00897-2. [DOI] [PubMed] [Google Scholar]
- [8].Ganglberger E, Barbara S, Scholl I, Wiedermann U, Baumann S, Hafner C, et al. Monovalent fusion proteins of IgE mimotopes are safe for therapy of type I allergy. FASEB J. 2001;15:2524–6. doi: 10.1096/fj.00-0888fje. [DOI] [PubMed] [Google Scholar]
- [9].Wachholz PA, Durham SR. Induction of ‘blocking’ IgG antibodies during immunotherapy. Clin Exp Allergy. 2003;33:1171–4. doi: 10.1046/j.1365-2222.2003.01765.x. [DOI] [PubMed] [Google Scholar]
- [10].Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–22. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- [11].Untersmayr E, Szalai K, Riemer AB, Hemmer W, Swoboda I, Hantusch B, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–61. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- [12].Bramswig KH, Knittelfelder R, Gruber S, Untersmayr E, Riemer AB, Szalai K, et al. Immunization with mimotopes prevents growth of carcinoembryonic antigen positive tumors in BALB/c mice. Clin Cancer Res. 2007;13:6501–8. doi: 10.1158/1078-0432.CCR-07-0692. [DOI] [PubMed] [Google Scholar]
- [13].Riemer AB, Untersmayr E, Knittelfelder R, Duschl A, Pehamberger H, Zielinski CC, et al. Active induction of tumor-specific IgE antibodies by oral mimotope vaccination. Cancer Res. 2007;67:3406–11. doi: 10.1158/0008-5472.CAN-06-3758. [DOI] [PubMed] [Google Scholar]
- [14].Hartl A, Kiesslich J, Weiss R, Bernhaupt A, Mostbock S, Scheiblhofer S, et al. Immune responses after immunization with plasmid DNA encoding Bet v 1, the major allergen of birch pollen. J Allergy Clin Immunol. 1999;103:107–13. doi: 10.1016/s0091-6749(99)70533-6. [DOI] [PubMed] [Google Scholar]
- [15].Weiss R, Hammerl P, Hartl A, Hochreiter R, Leitner WW, Scheiblhofer S, et al. Design of protective and therapeutic DNA vaccines for the treatment of allergic diseases. Curr Drug Targets Inflamm Allergy. 2005;4:585–97. doi: 10.2174/156801005774322171. [DOI] [PubMed] [Google Scholar]
- [16].Scheiblhofer S, Gabler M, Leitner WW, Bauer R, Zoegg T, Ferreira F, et al. Inhibition of type I allergic responses with nanogram doses of replicon-based DNA vaccines. Allergy. 2006;61:828–35. doi: 10.1111/j.1398-9995.2006.01142.x. [DOI] [PubMed] [Google Scholar]
- [17].Bauer R, Scheiblhofer S, Kern K, Gruber C, Stepanoska T, Thalhamer T, et al. Generation of hypoallergenic DNA vaccines by forced ubiquitination: preventive and therapeutic effects in a mouse model of allergy. J Allergy Clin Immunol. 2006;118:269–76. doi: 10.1016/j.jaci.2006.03.033. [DOI] [PubMed] [Google Scholar]
- [18].Fest S, Huebener N, Weixler S, Bleeke M, Zeng Y, Strandsby A, et al. Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 2006;66:10567–75. doi: 10.1158/0008-5472.CAN-06-1158. [DOI] [PubMed] [Google Scholar]
- [19].Rice J, Elliott T, Buchan S, Stevenson FK. DNA fusion vaccine designed to induce cytotoxic T cell responses against defined peptide motifs: implications for cancer vaccines. J Immunol. 2001;167:1558–65. doi: 10.4049/jimmunol.167.3.1558. [DOI] [PubMed] [Google Scholar]
- [20].Scheiblhofer S, Stoecklinger A, Gruber C, Hauser-Kronberger C, Alinger B, Hammerl P, et al. Gene gun immunization with clinically relevant allergens aggravates allergen induced pathology and is contraindicated for allergen immunotherapy. Mol Immunol. 2007;44:1879–87. doi: 10.1016/j.molimm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- [21].Weiss R, Scheiblhofer S, Freund J, Ferreira F, Livey I, Thalhamer J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine. 2002;20:3148–54. doi: 10.1016/s0264-410x(02)00250-5. [DOI] [PubMed] [Google Scholar]
- [22].Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–84. [PubMed] [Google Scholar]
- [23].Hartl A, Weiss R, Hochreiter R, Scheiblhofer S, Thalhamer J. DNA vaccines for allergy treatment. Methods. 2004;32:328–39. doi: 10.1016/j.ymeth.2003.08.014. [DOI] [PubMed] [Google Scholar]
- [24].Gabler M, Scheiblhofer S, Kern K, Leitner WW, Stoecklinger A, Hauser-Kronberger C, et al. Immunization with a low-dose replicon DNA vaccine encoding Phl p 5 effectively prevents allergic sensitisation. J Allergy Clin Immunol. 2006;118:734–41. doi: 10.1016/j.jaci.2006.04.048. [DOI] [PubMed] [Google Scholar]
- [25].Waldegrave W. Directive CEE 86/609. J Officiel Communautés. 1986;L358:1–28. [Google Scholar]
- [26].Riemer A, Scheiner O, Jensen-Jarolim E. Allergen mimotopes. Methods. 2004;32:321–7. doi: 10.1016/j.ymeth.2003.08.010. [DOI] [PubMed] [Google Scholar]
- [27].van Neerven RJ. The role of allergen-specific T cells in the allergic immune response: relevance to allergy vaccination. Allergy. 1999;54:552–61. doi: 10.1034/j.1398-9995.1999.t01-1-00092.x. [DOI] [PubMed] [Google Scholar]
- [28].Ganglberger E, Grunberger K, Sponer B, Radauer C, Breiteneder H, Boltz-Nitulescu G, et al. Allergen mimotopes for 3-dimensional epitope search and induction of antibodies inhibiting human IgE. FASEB J. 2000;14:2177–84. doi: 10.1096/fj.99-1000com. [DOI] [PubMed] [Google Scholar]
- [29].Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine. 2000;18:1725–9. doi: 10.1016/s0264-410x(99)00432-6. [DOI] [PubMed] [Google Scholar]
- [30].Oran AE, Robinson HL. DNA vaccines, combining form of antigen and method of delivery to raise a spectrum of IFN-gamma and IL-4-producing CD4+ and CD8+ T cells. J Immunol. 2003;171:1999–2005. doi: 10.4049/jimmunol.171.4.1999. [DOI] [PubMed] [Google Scholar]
- [31].Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–25. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Scheiblhofer S, Weiss R, Durnberger H, Mostbock S, Breitenbach M, Livey I, et al. A DNA vaccine encoding the outer surface protein C from Borrelia burgdorferi is able to induce protective immune responses. Microbes Infect. 2003;5:939–46. doi: 10.1016/s1286-4579(03)00182-5. [DOI] [PubMed] [Google Scholar]
- [33].Weiss R, Leitner WW, Scheiblhofer S, Chen D, Bernhaupt A, Mostbock S, et al. Genetic vaccination against malaria infection by intradermal and epidermal injections of a plasmid containing the gene encoding the Plasmodium berghei circumsporozoite protein. Infect Immun. 2000;68:5914–9. doi: 10.1128/iai.68.10.5914-5919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jensen-Jarolim E, Wiedermann U, Ganglberger E, Zurcher A, Stadler BM, Boltz-Nitulescu G, et al. Allergen mimotopes in food enhance type I allergic reactions in mice. FASEB J. 1999;13:1586–92. doi: 10.1096/fasebj.13.12.1586. [DOI] [PubMed] [Google Scholar]
- [35].Parmley SF, Smith GP. Filamentous fusion phage cloning vectors for the study of epitopes and design of vaccines. Adv Exp Med Biol. 1989;251:215–8. doi: 10.1007/978-1-4757-2046-4_21. [DOI] [PubMed] [Google Scholar]
- [36].Ito K, Shinohara N, Kato S. DNA immunization via intramuscular and intradermal routes using a gene gun provides different magnitudes and durations on immune response. Mol Immunol. 2003;39:847–54. doi: 10.1016/s0161-5890(03)00024-5. [DOI] [PubMed] [Google Scholar]