Summary
To investigate the role of aquaporin-mediated water transport during pollen grain germination and tube growth, Arabidopsis thaliana plasma membrane intrinsic proteins (PIPs) were expressed in pollen of Lilium longiflorum (lily).
Successful expression of AtPIPs in particle-bombarded lily pollen grains was monitored by co-expression with fluorescent proteins and single-cell RT-PCR, and by measuring the water permeability coefficient (Pos) in swelling assays using protoplasts prepared from transformed pollen grains and tubes.
Expression of AtPIP1;1 and AtPIP1;2 in pollen grains resulted in Pos values similar to those measured in nontransformed pollen grain protoplasts (6.65 ± 2.41 μm s−1), whereas expression of AtPIP2 significantly increased Pos (AtPIP2;1, 13.79 ± 6.38; AtPIP2;2, 10.16 ± 3.30 μm s−1). Transformation with combinations of AtPIP1 and AtPIP2 did not further enhance Pos. Native pollen tube protoplasts showed higher Pos values (13.23 ± 4.14 μm s−1) than pollen grain protoplasts but expression of AtPIP2;1 (18.85 ± 7.60 μm s−1) did not significantly increase their Pos values. Expression of none of the tested PIPs had any effect on pollen tube growth rates.
The ectopic expression of AtPIP2s in lily pollen increased the water permeability of the plasma membrane in pollen grains, but not in pollen tubes. The measured endogenous water permeability does not limit water uptake during tube growth, but has to be regulated to prevent tube bursting.
Keywords: aquaporin, fluorescent protein, pollen, protoplast, tip growth, water transport
Introduction
A mature pollen grain landing on a perceptive stigma rehydrates by taking up water from its environment. The hydrated pollen grain then starts to grow a pollen tube that penetrates the stigma tissue and delivers the sperm cells to the ovary for fertilization. Water uptake is important during at least two stages of pollen development: rehydration on the stigma and growth of the pollen tube. After the formation of a continuous lipid bilayer during rehydration (Crowe et al., 1989; Tiwari et al., 1990; Hoekstra et al., 1991), additional water has to be taken up across the plasma membrane to allow adjustment of cytosolic ion concentrations, for example hydrogen (H+), calcium (Ca2+) and potassium (K+), and turgor pressure. This early water uptake was postulated to be an initial step in pollen grain germination (Feijó et al., 1995). The hydration of Brassica pollen grains is mediated via plasma membrane intrinsic proteins (PIPs), and the PIP-dependent water permeability of papillar and subpapillar cells of the stigma, demonstrated by expression of PIP1 genes in these cells, has been suggested to be involved in the self-incompatibility process in Brassicaceae (Sarker et al., 1988; Ikeda et al., 1997; Marin-Olivier et al., 2000). These studies show the importance of aquaporins in the stigma tissue to control the supply of water. However, the situation on the male side, that is, water uptake by the pollen grain, remains less clear and contradictory results concerning the presence of PIPs in Brassica pollen have been published (Marin-Olivieret al., 2000; Dixit et al., 2001). Because of limitations in the availability of tools and genome sequences, the only comprehensive analysis of major intrinsic protein (MIP) gene expression was previously performed in Arabidopsis thaliana. However, Bock et al. (2006) could not detect any PIP transcripts using the ATH1 gene chip. Only tonoplast intrinsic proteins (TIP: AtTIP1;3, AtTIP5;1) and NOD 26-like intrinsic proteins (NIP: AtNIP4;1) were preferentially expressed in mature pollen. Both TIP genes had been found previously in an expression analysis of hydrated A. thaliana pollen grains together with a single PIP gene, AtPIP1;3 (Becker et al., 2003). The findings of both analyses are corroborated by the AtGen-Express plant organ data (www.genevestigator.ethz.ch), which do not indicate substantial expression of any PIP gene in A. thaliana pollen. Using the more sensitive RT-PCR, Bots et al. (2005) were able to detect two PIP mRNAs, NtPIP1;1 and NtPIP2;1, in tobacco (Nicotiana tabacum) pollen grains and tubes, but again at a much lower level than the corresponding transcripts detected in complete reproductive tissues such as anthers and pistils. Thus, in A. thaliana pollen no substantial transcription of PIP and even most MIP genes was detected. In Brassica and tobacco pollen there is very low transcription of PIP genes, as revealed by molecular biology studies.
However, physiological studies have provided substantial evidence for water transport during pollen grain germination and tube growth. Pollen tubes are one of the fastest growing plant cells and sufficient water uptake has to accommodate this increase in volume. A water uptake rate of 9.33 μm3 s−1 (c. 0.56 pl min−1) was estimated in a study on the hydrodynamics of growing Agapanthus umbellatus pollen tubes (Malhó & Pais, 1992) and volume changes were observed in tip-growing tobacco pollen tubes (Zonia et al., 2002, 2006). In addition, water uptake must be well regulated, as demonstrated by the finding that the turgor pressure of lily (Lilium longiflorum) pollen tubes adapted to the respective osmolality of the germination medium (Benkert et al., 1997), whereas sudden concentration changes immediately arrested tube growth and prevented Ca2+ influxes (Pierson et al., 1994). Despite this clear evidence for water uptake and osmoregulation during germination and tube growth, data on the water transport itself or on the specific role of aquaporin-mediated water transport are completely lacking to date.
To investigate a putative physiological role of aquaporins in water uptake and pollen tube growth, four well-characterized PIP genes of A. thaliana were transiently expressed in lily pollen by particle bombardment. Their effects on the water transport properties of protoplasts obtained from transformed pollen grains and tubes were measured in swelling assays and their effects on pollen tube growth rates were also determined.
Materials and Methods
Plant material, in vitro germination and protoplast preparation
Pollen grains of Lilium longiflorum Thunb. grown in a glasshouse under normal environmental conditions were collected from dehydrated anthers and used immediately for experiments or frozen in liquid N2 and stored at −80°C. No differences in germination frequency and tube morphology between fresh and frozen pollen grains were detected.
To measure tube growth rates, pollen grains were suspended in germination buffer (10% (w/v) sucrose, 1.6 mM H3BO3, 1 mM KCl, and 0.1 mM CaCl2, pH 5.6). For germination and tube growth measurements of transformed pollen, the isolation medium was carefully exchanged in a stepwise manner with germination medium. Tube growth was monitored by taking images every 15 s with a video-equipped microscope (Axiovert 135; Zeiss, Oberkochen, Germany). Pollen tube lengths were measured using Meta Imaging Series 6.3 software (Visitron Sytems, Puchheim, Germany).
Protoplasts from control and transformed lily pollen grains and tubes were prepared as described previously (Griessner & Obermeyer, 2003). Briefly, lily pollen grains from one anther were incubated in 2.5 ml of isolation medium (IsoMed: 680mM mannitol, 5mM CaCl2, 10 mM KCl, 0.5 mM ascorbic acid and 10 mM 2-morpholino-ethanesulfonic acid (MES) adjusted with 1,3-bis(tris(hydroxymethyl)methylamino)propane (BTP) to pH 6.0) containing the following enzyme concentrations: 24 U ml−1 cellulase (Onozuka; Serva, Heidelberg, Germany), 2 U ml−1 pectinase (Sigma, Taufkirchen, Germany) and 2.6 U ml−1 macerozyme (Serva). Pollen grains were incubated in the dark, with continuous gentle shaking at room temperature. Released protoplasts were pipetted into a Petri dish with fresh, ice-cold IsoMed without enzymes using a Pasteur pipette with a tip diameter of 200–300 μm. This manual washing procedure was repeated twice and protoplasts were always kept on ice. For preparation of pollen tube protoplasts, transformed, fluorescent pollen grains were manually collected, re-suspended in germination medium, and incubated at room temperature. Germinated pollen grains were then suspended in an enzyme solution (IsoMed with 22 U ml−1 cellulase and 11.2 U ml−1 macerozyme) and incubated at room temperature for 30 min. The fluorescent pollen tube protoplasts were washed like the pollen grain protoplasts. All collected protoplasts were kept on ice and used within 1–4 h for swelling experiments. No regeneration of cell walls could be observed under these conditions.
Construction of expression plasmids
To generate the plasmid pLAT52pA2, nucleotides −602 (SalI) to −5 with respect to the ATG start codon of the tomato (Lycopersicon esculentum) LeLAT52 promoter (accession number X15855; Twell et al., 1990) were fused in a pBluescript KS (+) vector background (Stratagene, La Jolla, CA, USA) with the SacI–NotI vector cassette at the 5′-side of the promoter, a polylinker sequence 5′-ACCATGGGGATCCGAATTCGATATCAAGCTTATCGATTCTAGACTGCAG followed by the nopaline synthase polyadenylation sequence (NOS; 252 bp), a regenerated BglII site and the vector-derived XhoI–KpnI cassette. The coding region of a synthetic green fluorescent protein (GFP) gene and the same NOS sequence (Chiu et al., 1996) were fused with the LeLAT52 promoter fragment described above to create the plasmid pLat52::sGFP.
To obtain pSUC3::sGFP, pSUC3::ECFP, and pSUC3::EYFP the coding sequences of the three fluorescent proteins were amplified by PCR with a 5′-BsiEI and a 3′-BamHI adaptor and ligated into the SacII and BglII sites of pCL23 (Meyer et al., 2004), removing all four restriction sites from the final construct; in addition the 5′-NcoI site (mutation in the oligo for PCR amplification) and the 3′-SacI and XbaI sites (restriction with SacI followed by T4 DNA polymerase and re-ligation) were eliminated.
Using 5′-GGGGACTCGAGCTTGGTAAGAAGAACTCTATCAC and 5′-GGGGAGAGCT CCCGACTAGTAACATAGATGAC the complete expression cassettes of pSUC3::sGFP, pSUC3::ECFP, and pSUC3::EYFP were amplified and ligated head-to-head into pLAT52pA2 digested with SacI and SalI to obtain plasmid vectors pSUC3::XFP-LAT52pA. These plasmid vectors harbor a polylinker for insertion of any open reading frame of interest under the control of the pollen-specific LeLAT52 promoter and a complete expression cassette for expression of either of the fluorescent proteins under the control of the pollen-expressed AtSUC3 promoter. In this study, the coding regions of AtPIP1;1 and AtPIP1;2 were amplified by PCR from cDNA clones, restricted with BamHI/HindIII and NcoI/EcoRI, respectively, and ligated into pSUC3::ECFP-LAT52pA to generate pSUC3::ECFP-LAT52-AtPIP1;1 and pSUC3::ECFP-LAT52-AtPIP1;2. Similarly, AtPIP2;1 and AtPIP2;2 were amplified, and cut with BamHI/HindIII and BspHI/HindIII to create pSUC3::EYFP-LAT52-AtPIP2;1 and pSUC3::EYFP-LAT52-AtPIP2;2.
Transformation of lily pollen
The pollen grains of one anther were rehydrated for 20 min in 1 ml of IsoMed, washed three times with the same medium and finally re-suspended in 50 μl of IsoMed. The pollen grain suspension was carefully deposited in the middle of a filter paper and placed at the bottom of a well (six- or 24-well tissue culture plate; Greiner, Kremsmünster, Austria). Usually, the pollen grains of at least two anthers were transformed by particle bombardment using a Helios Gene Gun (BioRad, Hercules, CA, USA). Gold particles were coated with plasmids according to the manufacturer’s instructions. The DNA loading ratio was 2 μg of DNA per mg of gold beads, and the microcarrier loading quantity (MLQ) was 0.5 mg of microcarrier per shot. For double-coating experiments, a ratio of 1 : 1 (w/w) for the two plasmids was used. The optimal bombardment conditions for lily pollen grain transformation were 1.6-μm gold beads, a helium pressure of 200 psi and a distance of 2 cm between the gene gun spacer and the target. Immediately after the bombardment, the pollen grains were suspended in 2.5 ml of fresh IsoMed and incubated in small Petri dishes in the dark at room temperature for 16–18 h, unless otherwise stated. The transformation efficiency for each bombardment experiment was determined by counting the percentage of fluorescent pollen grains in each Petri dish.
Fluorescence microscopy
The transformed pollen grains were screened for the expression of the respective fluorescent protein using an inverted microscope (Axiovert 135) equipped with a CCD video camera (SPOT Insight; Visitron Systems, Puchheim, Germany) and the appropriate filter settings (AHF, Tübingen, Germany) for GFP (ex, 470 ± 20 nm; dichroic mirror, 495 nm; em, 525 ± 25 nm), yellow fluorescent protein (YFP) (ex, 500 ± 10 nm; dichroic, 515 nm; em, 535 ± 15 nm), and cyan fluorescent protein (CFP) (ex, 436 ± 5 nm; dichroic, 460 nm; em, 470 ± 15 nm).
Protoplast swelling assay and determination of Pos
To determine the permeability coefficient (Pos) of pollen grain and tube protoplasts obtained from control as well as transformed pollen, protoplasts were pipetted into hypoosmolar medium (same composition as the isolation medium but with osmolality set to 400–500 mOsmol), and images were acquired at a sampling rate of 0.25 Hz for 450–600 s with the video-equipped, inverted microscope, and finally analysed using the Meta Imaging software. The osmolalities of all media were determined with an osmometer (Gonotec, Berlin, Germany) for each experiment. The diameter D of a swelling protoplast was measured and the corresponding volume was calculated as V = (πD3)/6. Data processing and the calculation of Pos and the relative nonosmotic volume β were performed as described previously (Sommer et al., 2007). Briefly, the measured volume values were normalized and transformed to relative osmotic volume values by considering β, according to the following relation:
| Eqn 1 |
with and . The subscripts ‘0’ and ‘f’ (Eqns 2, 4) refer to initial (t = 0 s) and final (steady-state) values, respectively, and the superscript ‘os’ to osmotic variables as opposed to measured variables. The value of the relative nonosmotic volume β was inferred for each measured protoplast from the corresponding volume transient using the following equation:
| Eqn 2 |
In a second step, the values of the relative osmotic volume were fitted with Eqn 3 and the fit parameter k was finally used to calculate the osmotic water permeability coefficient Pos (Eqn 4; see also Sommer et al., 2007):
| Eqn 3 |
| Eqn 4 |
with the following notations for α: . Fitting routines were performed with SigmaPlot 9 software (Jandel Scientific, Erkrath, Germany).
Statistical analysis
Mean values and standard deviations (SDs) were calculated. Significance levels were determined using SigmaStat 3 or SigmaPlot 9 software ( Jandel Scientific), performing either an unpaired Student’s t-test or a Mann–Whitney rank sum test as indicated. Two data groups were designated as significantly different for P < 0.01.
Single-cell RT-PCR
Detection of the mRNAs of AtPIP1;1 in individual, transformed pollen grain protoplasts was carried out according to Gehwolf et al. (2002). First an AtPIP1;1-specific, digoxigenin-labelled probe was generated by amplification of a 403-bp region of the ATPIP1;1 plasmid with the following primer pair: AtPIP1_1_fwd (5′-TGCTGAGATCATTGGCACA-3′) and NOSpA_rev (5′-AAGACCGGCAACAGGATT-3′). The specificity of the probe and the hybridization conditions were optimized by hybridization of the probe with digested control plasmids containing AtPIP1;1 (BamHI/HindIII), AtPIP2;1 (BamHI/HindIII), and AtPIP1;2 (NcoI/EcoRI) sequences. Single protoplasts were collected after a swelling experiment, pipetted into PCR tubes with 1 μl of protoplast medium plus 20 U RNasin (Fermentas, St Leon-Rot, Germany), and frozen in liquid N2 to break the protoplast. Reverse transcription was performed with the specific reverse primer NOSpA-rev and products were subsequently amplified by PCR with the AtPIP1_1_fwd and NOSpA_rev primers. Amplified products were electrophoresed in a 1% agarose gel, transferred onto a membrane and detected with the digoxigenin-labelled probe as follows: hybridization at 60°C overnight, 2 × 5 min washing at room temperature with 2 × saline sodium citrate (SSC) + 0.1% sodium dodecyl sulphate (SDS) and washing at 72°C for 20 min twice with 0.1 × SSC + 0.1% SDS. After washing in maleate-Tween 20 buffer the hybridized probe was detected with anti-digoxigenin antibody conjugated with horseradish peroxidase (Roche Diagnostics, Vienna, Austria) by chemiluminescence (ECL; GE-Healthcare, Vienna, Austria).
Results
Optimization of the bombardment conditions
To determine the optimal conditions for the transient transformation of lily pollen, the particle bombardment parameters were changed by a step-by-step procedure to achieve a high transformation rate while leaving pollen germination unaffected and keeping loss of material to a minimum. In the first step, the gold particle size (1.6 μm) and the MLQ (0.25 mg per shot) were kept constant while the helium pressure (100, 150, 200 and 400 psi) and distance to the target (0 and 2 cm) were varied. At pressures higher than 200 psi and no additional distance (0 cm) between the gene gun and the pollen we noted high sample loss and considerable damage of the pollen grains for all the pressures used. In contrast, a helium pressure of 100 psi led to an unacceptably low transformation rate. Helium pressures of 200 and 150 psi and a distance of 2 cm to the target were tested while varying the size of the gold particles (1 and 1.6 μm) and the MLQ (0.25 and 0.5 mg per shot). The combination that gave the best and most reliable results was 0.5 mg of gold per shot, a particle size of 1.6 μm, a distance of 2 cm and a helium pressure of 200 psi, and this combination was therefore used throughout this study.
Transient expression of fluorescent proteins in lily pollen
To analyse the effect of particle bombardment and the transient expression of fluorescent proteins on water permeability, pollen grains were transformed with plasmids pLAT52::sGFP and pSUC3::sGFP containing the GFP sequence under the control of two promoters. The LeLAT52 (Twell et al., 1990) and AtSUC3 (Meyer et al., 2004) promoters had previously been shown to direct expression in pollen of tomato and A. thaliana, respectively. GFP-specific fluorescence could be observed 16–24 h after transformation, indicating that both promoters are also active in pollen grains of the monocot plant L. longiflorum, and the observed fluorescence intensity was independent of the promoter used (data not shown). Pollen grain protoplasts that expressed GFP under the control of the AtSUC3 and LeLAT52 promoters showed permeability coefficient Pos values of 5.41 ± 1.50 and 6.97 ± 2.23 μm s−1, respectively, and were not significantly different (P > 0.05, Student’s t-test) from pollen grain protoplasts bombarded with noncoated gold particles (Fig. 1, no plasmid: 7.44 ± 2.87 μm s−1). The Pos values of all protoplasts presented in Fig. 1 ranged from 3.5 to 11 μm s−1, giving a mean value of 6.65 ± 2.41 μm s−1, which is similar to that of native, untreated lily pollen grain protoplasts (6.59 ± 2.26 μm s−1; Sommer et al., 2007). Therefore, neither the bombardment itself, nor the expression of a fluorescent protein, nor the subsequent incubation affected the water permeability of the plasma membrane of the protoplasts.
Fig. 1.
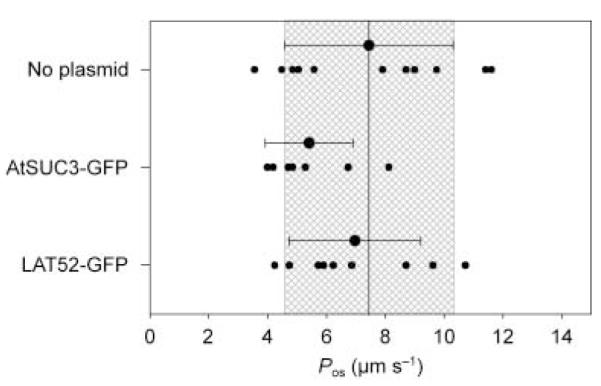
Water permeability coefficient (Pos) measured in lily (Lilium longiflorum) control pollen grain protoplasts (only gold particles, not plasmid coated) or protoplasts obtained from pollen grains bombarded with gold beads coated with AtSUC3::GFP and LeLAT52::GFP plasmids. Small data points indicate individual experiments. Large data points are mean ± SD. The mean Pos value of controls is indicated by the solid line; the grey box indicates the SD of control values.
Expression of AtPIPs in lily pollen
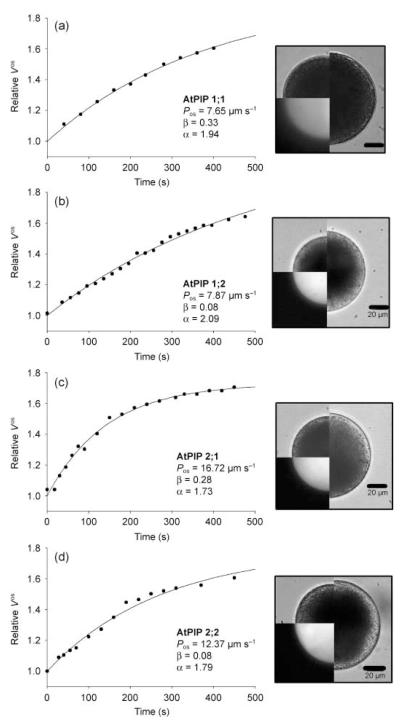
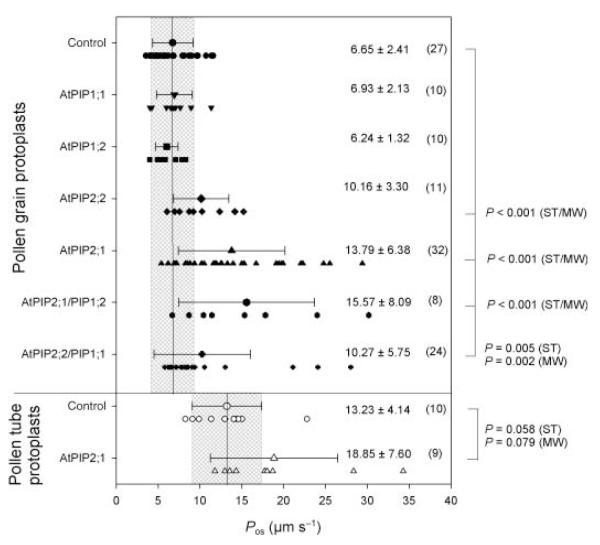
To investigate the putative role of aquaporins during water transport across the plasma membrane and subsequently in pollen tube growth, pollen grains were bombarded with plasmids of the type pSUC3::xFP_LAT52::PIPy;z (see the Materials and Methods; xFP = CFP or YFP and PIPy;z = PIP1;1, PIP1;2, PIP2;1 or PIP2;2) that allow the expression of a fluorescent protein under the control of the AtSUC3 promoter and the expression of the respective PIP under the control of the LeLAT52 promoter. The PIP genes were chosen because they are highly expressed in A. thaliana plants (Kammerloher et al., 1994; Jang et al., 2004) and were found to confer a slight (PIP1;1 and PIP1;2) or strong (PIP2;1 and PIP2;2) enhancement of water permeability in the heterologous oocyte expression system (Kammerloher et al., 1994). Bright field and fluorescence images of the respective protoplasts, for which a volume transient was measured, were taken at the start (t0) as well as at the end (tfinal) of a swelling episode and clearly show the expression of the respective fluorescent protein and the increase in protoplast volume (Fig. 2). Typical time-dependent increases in the relative osmotic volume () of pollen grain protoplasts bombarded with plasmids for the expression of AtPIP1;1/CFP and AtPIP1;2/CFP are shown in Fig. 2a and b, respectively. Although both protoplasts showed CFP fluorescence, indicating their transformation, their Pos values (7.65 μm s−1 for AtPIP1;1 and 7.87 μm s−1 for AtPIP1;2) were not different from those of control protoplasts. However, pollen grain protoplasts expressing PIP2;1/YFP or PIP2;2/YFP did show an increase in Pos values (16.72 and 12.37 μm s−1, respectively), indicating an increase in the water permeability of the protoplast plasma membrane upon PIP2 expression (Fig. 2c,d). This result is confirmed by a statistical comparison of the means of the respective Pos values obtained from protoplasts expressing members of the PIP1 or PIP2 family (Fig. 3): pollen grain protoplasts transformed with PIP2;1 (13.79 ± 6.38 μm s−1) and PIP2;2 (10.16 ± 3.30 μm s−1) showed a significant increase in Pos, whereas the values for pollen grain protoplasts expressing PIP1;1 (6.93 ± 2.13 μm s−1) and PIP1;2 (6.24 ± 1.32 μm s−1) were within the range of the controls.
Fig. 2.
Typical volume transients (swelling curves) of protoplasts obtained from lily (Lilium longiflorum) pollen grains transformed with Arabidopsis thaliana plasma membrane intrinsic protein 1;1 (AtPIP1;1)/cyan fluorescent protein (CFP) plasmids (a), AtPIP1;2/CFP plasmids (b), AtPIP2;1/yellow fluorescent protein (YFP) plasmids (c), or AtPIP2;2/YFP plasmids (d). The calculated water permeability coefficient (Pos) and the respective parameters α and β (see Materials and Methods) are given for each individual protoplast. The images show the protoplast at t = 0 min as bright field images (upper left quarter) and fluorescence images (lower left quarter). The right half of the image shows the protoplast at the end of the swelling curve (tfinal). Bars, 20 μm.
Fig. 3.
Summary of water permeability coefficient (Pos) values measured in control and transformed lily (Lilium longiflorum) pollen grain protoplasts as well as in control and transformed pollen tube protoplasts. Individual Pos values and mean ± SD are presented. Solid lines and grey boxes indicate the mean and the corresponding SD of the respective control Pos values. The respective mean ± SD of Pos values for each treatment is given in μm s−1 and the number in brackets indicates the number of individual protoplasts measured. Pos values significantly different from control data are indicated, with significance values (P) calculated using Student’s t-test (ST) and the Mann–Whitney test (MW).
To confirm the transcription of AtPIP1;1 in cells showing the expression of the corresponding fluorescent reporter (CFP) without an effect on the swelling behaviour, single-cell RT-PCR experiments on individual pollen grain protoplasts transformed with AtPIP1;1/CFP were performed. A PIP1;1-specific signal of the expected size could be observed in the transformed, but not in the nontransformed, control pollen grain protoplasts (Fig. 4), indicating the successful transcription of AtPIP1;1. Immunological detection of the AtPIP1 protein was not possible because of the lack of an antibody recognizing AtPIP1 proteins specifically in lily protoplasts and in a microsomal fraction of lily pollen (data not shown). Therefore, no definite conclusion can be drawn regarding the effect of PIP1 proteins on water permeability.
Fig. 4.
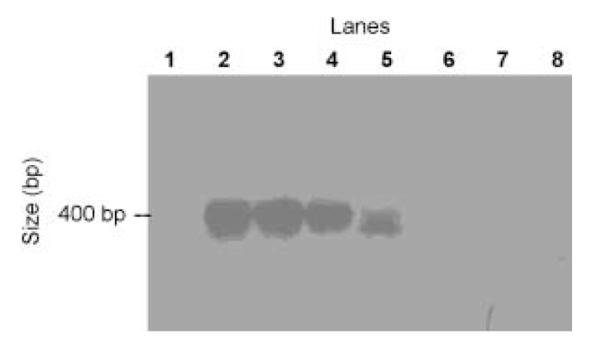
Single-cell RT-PCR of Arabidopsis thaliana plasma membrane intrinsic protein 1;1 (AtPIP1;1)-transformed and control pollen grain protoplasts from lily (Lilium longiflorum). Fluorescent as well as control pollen grain protoplasts were collected and immediately frozen in liquid nitrogen. cDNA was generated by reverse transcription with AtPIP1;1-specific reverse primer, followed by PCR with an AtPIP1;1-specific primer pair. Amplified product was separated by agarose gel electrophoresis, blotted onto a membrane and detected using a digoxigenin-labelled specific AtPIP1;1 probe. Lane 1, water control; lanes 2–5, individual, transformed protoplasts; lanes 6–8, nontransformed protoplasts.
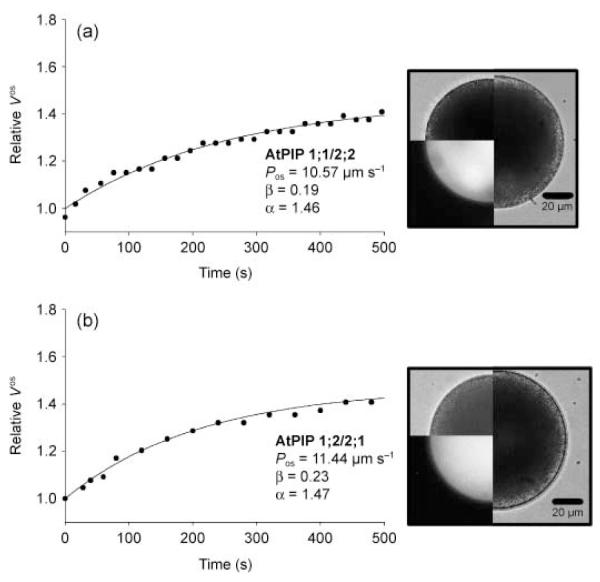
Recent observations indicate that PIP1 and PIP2 proteins might interact (Fetter et al., 2004; Zelazny et al., 2007) and a synergistic enhancement of water permeability has been shown in the heterologous oocyte expression system (Fetter et al., 2004; Temmei et al., 2005). Co-expression analyses based on public array data indicated that AtPIP1;1 and AtPIP2;2 as well as AtPIP1;2 and AtPIP2;1 show the highest correlation factors for PIP gene expression (O. Da Ines & A. R. Schäffner, unpublished; Arabidopsis Co-expression Data Mining Tool ACT (Manfield et al., 2006)). Therefore, these combinations were chosen for co-expression in lily pollen grains. For co-transformation, the gold microcarriers were double-coated with the respective PIP1/CFP and PIP2/YFP plasmids. It should be noted that the fluorescence of CFP was faint when CFP was co-expressed with YFP as long as equimolar ratios were used, an observation also made in transformed mammalian cells (pers. comm., Markus Ritter, Paracelsus Medical University, Salzburg, Austria). Indeed, the spectroscopic properties of the two fluorescent proteins are very different: the molar extinction coefficient of YFP is almost three times higher than that of CFP, and thus YFP has a higher quantum efficiency (Dixit et al., 2006). At a weight ratio of 4 : 1 for CFP:YFP plasmids, the fluorescence intensities of the two proteins were comparable (data not shown). Nevertheless, a 1 : 1 plasmid ratio was used in the co-transformation of lily pollen grains, because we were interested in equal expression of PIP1 and PIP2 driven by the same promoter. Simultaneous transformation of pollen grains with a PIP1 and a PIP2 did not further increase water permeability (Fig. 5). Typical swelling experiments for the combinations AtPIP1;1 with AtPIP2;2 and AtPIP1;2 with AtPIP2;1 resulted in Pos values of 10.57 and 11.44 μm s−1, respectively (Fig. 5a,b). The Pos values were in the range of those measured in protoplasts expressing the respective PIP2 protein alone, as summarized in Fig. 3, which could be confirmed by statistical analysis using Student’s t-test or the Mann–Whitney rank sum test.
Fig. 5.
Swelling curves of protoplasts obtained from lily (Lilium longiflorum) pollen grains co-transformed with Arabidopsis thaliana plasma membrane intrinsic protein 1;1 (AtPIP1;1)/AtPIP2;2 (a) and AtPIP1;2/AtPIP2;1 (b). The images show the protoplast at t = 0 min as bright field images (upper left quarter) and as fluorescence images (lower left quarter). The right half of the image shows the protoplast at the end of the swelling curve (tfinal). Bars, 20 μm.
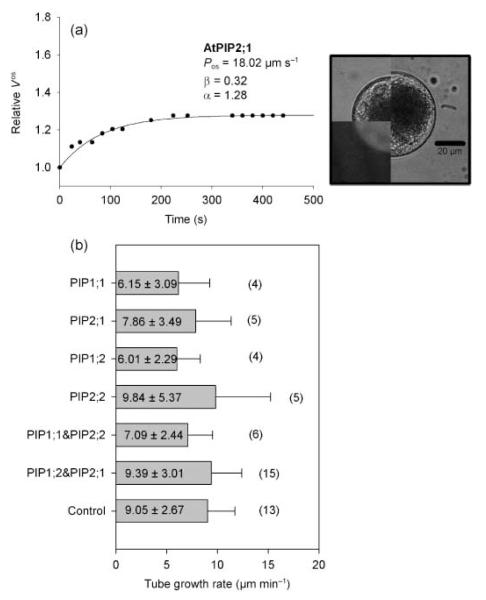
Protoplasts obtained from transformed pollen tubes also showed fluorescence and could be subjected to swelling assays. The volume transient of a pollen tube protoplast transformed with AtPIP2;1 is shown in Fig. 6a; this gave a Pos value of 18.02 μm s−1. The mean Pos value for transformed pollen tube protoplasts (18.85 ± 7.60 μm s−1) was not significantly different (P = 0.058) from that of control pollen tube protoplasts (13.23 ± 4.14 μm s−1; Fig. 3).
Fig. 6.
Typical swelling curve of a pollen tube protoplast transformed with Arabidopsis thaliana plasma membrane intrinsic protein 2;1 (AtPIP2;1) (a) and pollen tube growth rates measured in pollen tubes of control and transformed pollen grains (b). The images show the protoplast at t = 0 min as bright field images (upper left quarter) and as fluorescence images (lower left quarter). The right half of the image shows the protoplast at the end of the swelling curve (tfinal). Numbers in brackets give the number of individual experiments whereas the numbers in the boxes give the mean growth rate ± SD in μm min−1. Bar, 20 μm.
Growth of pollen tubes expressing AtPIPs
To determine whether there was an effect of the AtPIPs on pollen tube growth, the successfully transformed, fluorescent pollen grains were collected manually and carefully suspended in germination medium. Despite the long incubation time necessary for the expression of the fluorescent protein, the pollen grains started to germinate after a longer lag-phase (2–3 h) and transformed pollen tubes showed the same morphology as the control tubes (Supporting Information Fig. S1). The mean tube growth rates of pollen tubes transformed with either AtPIP1 or AtPIP2 plasmids ranged between 6 and 10 μm min−1 and were similar to that of control pollen tubes (9.05 ± 2.67 μm min−1; Fig. 6b).
Discussion
Expression and activity of AtPIPs in lily pollen
Transformation of lily pollen grains by particle bombardment with control gold beads or gold beads coated with plasmids containing expression cassettes for fluorescent proteins (CFP, GFP and YFP) did not affect the germination of pollen grains, pollen tube morphology or the water permeability coefficient Pos of the plasma membrane. This finding is in agreement with other reports showing that GFP expression did not alter pollen germination frequency or pollen tube growth rates (Keller & Hamilton, 1998; Hudson & Stewart, 2004). Protoplasts prepared from pollen grains expressing only fluorescent proteins exhibited similar water permeability coefficient values (6.65 ± 2.41 μm s−1) to protoplasts derived from nonbombarded pollen grains (6.59 ± 2.26 μm s−1; Sommer et al., 2007).
In contrast, expression of A. thaliana PIP2;1 and PIP2;2 increased water permeability in pollen grain protoplasts, whereas PIP1;1 and PIP1;2 did not. This result is in accordance with studies in heterologous, nonplant expression systems (Xenopus oocytes) in which no or lower water transport activity was detected when PIP1s were expressed (A. thaliana PIPs: Kammerloher et al., 1994; tobacco PIPs: Biela et al., 1999; Bots et al., 2005; maize (Zea mays) PIPs: Fetter et al., 2004; Brassica PIPs: Dixit et al., 2001; Samanea saman PIPs: Moshelion et al., 2002). Therefore, this study provides a proof-of-principle that pollen grain protoplasts are suitable for use as a plant-based expression system to test the functionality of putative aquaporins, in view of the simple handling procedures required, their ability to become transformed by particle bombardment and, most importantly, their low endogenous water permeability which was postulated as a prerequisite for ‘homologous’ expression systems for plant PIPs (Maurel, 2007).
As the functional activity of AtPIP2s observed in the pollen grain protoplasts is an unequivocal proof of their correct targeting to the plasma membrane, we did not investigate their localization in particular. PIPs can only contribute to water transport if they are incorporated into the plasma membrane of pollen grain protoplasts. Pollen tubes, where ectopic AtPIP2 expression did not further increase water permeability, developed from transformed pollen grains, still representing the same cells observed 2 h later. Therefore, the capability to locate the ectopic AtPIP2 to the plasma membrane will probably be retained. Indeed, the plasma membrane localization of PIP2 proteins was previously reported in several cell types with PIPs of Z. mays expressed in Xenopus oocytes and Z. mays mesophyll cells (Fetter et al., 2004; Zelazny et al., 2007) and with PIPs of A. thaliana root cells (Boursiac et al., 2005) by using GFP::PIP fusion proteins. However, we cannot exclude the possibility of post-translational modifications that may cause an inactivation of the PIPs in the plasma membrane of pollen tubes.
In contrast to Xenopus oocyte experiments, in which co-expression of PIP1s together with PIP2s from Z. mays further increased the water permeability of the oocytes (Fetter et al., 2004), we observed that co-expression of AtPIP1;2 with AtPIP2;1 or AtPIP1;1 with AtPIP2;2 did not increase the Pos values of lily pollen grain protoplasts compared with pollen grain protoplasts expressing the respective PIP2 alone. Localization of the ZmPIPs expressed in maize mesophyll protoplasts had revealed an interaction between PIP1 and PIP2: under these conditions, the preferentially endoplasmic reticulum (ER)-localized ZmPIP1s re-localized to the plasma membrane in the presence of ZmPIP2s (Zelazny et al., 2007). However, the effect on the water permeability of transformed maize mesophyll protoplasts was less clear: an increase in Pos was detected in mesophyll protoplasts expressing mCFP::ZmPIP2;1 whereas variable values were obtained upon co-expression with mYFP::ZmPIP1;2 (Zelazny et al., 2007). In the present study, the lack of an additive effect on water permeability upon co-expression of PIP1s and PIP2s in pollen protoplasts has several possible explanations: (1) the two AtPIPs do not interact although they harbour the amino acid residues, at the expected positions, proposed by Fetter et al. (2004) to be responsible for the interaction between PIP isoforms in oocytes; (2) they do interact but plant-specific regulation may prevent a further increase in water permeability, or (3) an endogenous lily pollen PIP1 interacts with the expressed AtPIP2 and forms a functional water channel in the plasma membrane, increasing water permeability. Additional expression of AtPIP1 cannot increase the water permeability any further because of the limiting amount of expressed AtPIP2s necessary for functional water channels.
Nevertheless, the endogenous water permeability of the pollen grain plasma membrane increased during pollen grain germination and subsequent tube growth, as indicated by the twofold enhancement of the measured Pos values of tube protoplasts (c. 6.5 μm s−1 for the pollen grain and 13 μm s−1 for the pollen tube). We can only speculate on the molecular background of this observation: there may be an increase in the translation rate of endogenous aquaporins, or their activity might be increased by post-translational regulation, or synergisms between different endogenous lily PIP isoforms may result in higher water permeability.
Water transport and pollen tube growth
The Pos values measured in this study can be used to estimate whether the measured rate of water uptake may account for the volume increase during tube growth. Pollen tubes grow by tip growth, showing a constant tube diameter, and can reach a length of up to 10 cm depending on the respective style length. This dramatic increase in cell volume has to be complemented mainly by the uptake of water. The rate of volume increase corresponding to the average pollen tube growth rate of 10 μm min−1 is 1.1 pl min−1 as calculated from the geometry of a lily pollen tube with a representative diameter of 12 μm.
Using the measured Pos values, water influxes can be calculated according to the equations presented by Steudle (1989) and Zimmermann (1989), resulting in a maximal water influx of about 110 pl min−1 for a pollen grain with a 500-μm-long pollen tube. This water influx is more than sufficient to allow the observed volume increase that occurs during tube growth and has to be tightly regulated by adjustment of the osmotic potential gradient across the plasma membrane to prevent bursting of the growing pollen tube. However, net water uptake might be reduced by allowing water efflux at a region different from the sites of water influx, as postulated by Zonia et al. (2006) and Zonia & Munnik (2007). This is, however, only possible if one assumes reversed driving force directions for water flux at different regions of the large, longitudinal pollen tube, which have not been measured to date.
In addition to their role as water channels, aquaporins might have a role in osmoregulation by sensing the pressure and/or osmotic gradient across the plasma membrane (Hill et al., 2004). Accordingly, in pollen, aquaporins may also function as osmosensors, preventing the growing tube from bursting, and thus, as part of the regulatory network, control pollen tube growth and contribute to the concerted action of the cellular processes necessary for tip growth (Feijó et al., 1995; Holdaway-Clarke & Hepler, 2003).
In conclusion, expression of A. thaliana PIPs in lily pollen showed that members of the PIP1 subfamily did not confer enhanced water channel activity, whereas members of the PIP2 subfamily clearly did. The water permeability of the plasma membrane of unmodified pollen tubes is higher than the water permeability of the plasma membrane of the pollen grain and, interestingly, the ectopic expression of AtPIP2s did not further enhance the water permeability of pollen tubes. The molecular basis of the physiological increase in Pos during pollen grain germination and tube growth, for example, differential expression or regulation of endogenous lily PIPs, will be a matter for future investigations. Nevertheless, the calculated water uptake rates and the fact that expression of AtPIPs did not increase pollen tube growth rates suggest that water permeability and the resulting water influx across the plasma membrane are not limiting pollen tube growth, but have to be tightly regulated to prevent pollen tube bursting.
Supplementary Material
Typical pollen tubes of control and transformed lily pollen are growing in germination medium.
Acknowledgements
We thank David Twell (University of Leicester, UK) and Christian Lauterbach as well as Norbert Sauer (University of Erlangen, Germany) for the gifts of the pollen-specific promoters LeLAT52 and AtSUC3, respectively. The work was partially funded by grants to GO (FWF, P17227-B03) and ARS (DFG priority programme 1108/Scha 454/8).
Footnotes
Supporting Information Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than about missing material) should be directed to the New Phytologist Central Office.
References
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijo J. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology. 2003;133:713–725. doi: 10.1104/pp.103.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkert R, Obermeyer G, Bentrup F-W. The turgor pressure of growing lily pollen tubes. Protoplasma. 1997;198:1–8. [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. The Nicotiana tabacum plasma membrane NtAQP1 is mercury-insensitive and permeable for glycerol. Plant Journal. 1999;18:565–570. doi: 10.1046/j.1365-313x.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi K, Twell D, Sze H. Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiology. 2006;140:1151–1168. doi: 10.1104/pp.105.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani C. PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. Journal of Experimental Botany. 2005;56:113–121. doi: 10.1093/jxb/eri009. [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu D-T, Sorieul M, van den Dries N, Maurel C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology. 2005;139:790–805. doi: 10.1104/pp.105.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Current Biology. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Crowe JA, Hoekstra FA, Crowe LM. Membrane phase transitions are responsible for imbibitional change in dry pollen. Proceedings of the National Academy of Sciences, USA. 1989;86:520–523. doi: 10.1073/pnas.86.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Cyr R, Gilroy S. Using intrinsically fluorescent proteins for plant cell imaging. Plant Journal. 2006;45:599–615. doi: 10.1111/j.1365-313X.2006.02658.x. [DOI] [PubMed] [Google Scholar]
- Dixit R, Rizzo C, Nasrallah ME, Nasrallah JB. The Brassica MIP-MOD gene encodes a functional water channel that is expressed in the stigma epidermis. Plant Molecular Biology. 2001;45:51–62. doi: 10.1023/a:1006428007826. [DOI] [PubMed] [Google Scholar]
- Feijó JA, Malhó R, Obermeyer G. Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma. 1995;187:155–167. [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. The Plant Cell. 2004;16:215–228. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehwolf R, Griessner M, Pertl H, Obermeyer G. First patch, then catch: measuring the activity and the mRNA transcripts of a proton pump in individual Lilium pollen protoplasts. FEBS Letters. 2002;512:152–156. doi: 10.1016/s0014-5793(02)02246-9. [DOI] [PubMed] [Google Scholar]
- Griessner M, Obermeyer G. Characterization of whole-cell K+ currents across the plasma membrane of pollen grain and pollen tube protoplasts of Lilium longiflorum. Journal of Membrane Biology. 2003;193:99–108. doi: 10.1007/s00232-002-2011-7. [DOI] [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? Journal of Membrane Biology. 2004;197:1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Crowe JH, Crowe LM. Effect of sucrose on phase behaviour of membranes in intact pollen of Typha latifolia L., as measured with Fourier transform infrared spectroscopy. Plant Physiology. 1991;97:1073–1079. doi: 10.1104/pp.97.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T, Hepler PK. Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- Hudson LC, Stewart CN. Effects of pollen-synthesized fluorescent protein on pollen grain fitness. Sexual Plant Reproduction. 2004;17:49–53. [Google Scholar]
- Ikeda S, Nasrallah JB, Dixit R, Preiss S, Nasrallah ME. An aquaporin-like gene required for the Brassica self-incompatibility response. Science. 1997;276:1564–1566. doi: 10.1126/science.276.5318.1564. [DOI] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology. 2004;54:713–725. doi: 10.1023/B:PLAN.0000040900.61345.a6. [DOI] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schäffner AR. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant Journal. 1994;6:187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Keller NL, Hamilton DA. Transient expression of the green fluorescent protein in pollen. Sexual Plant Reproduction. 1998;11:163–165. [Google Scholar]
- Malhó R, Pais MSS. Kinetics and hydrodynamics of Agapanthus umbellatus pollen tube growth: a structural and stereological study. Sexual Plant Reproduction. 1992;5:163–168. [Google Scholar]
- Manfield IW, Jen CH, Pinney JW, Michalopoulos I, Bradford JR, Gilmartin PM, Westhead DR. Arabidopsis Co-expression Tool (ACT): web server tools for microarray-based gene expression analysis. Nucleic Acids Research. 2006;34:504–509. doi: 10.1093/nar/gkl204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Olivier M, Chevalier T, Fobis-Loisy I, Dumas C, Gaude T. Aquaporin PIP genes are not expressed in the stigma papillae in Brassica napus. Plant Journal. 2000;24:231–240. doi: 10.1046/j.1365-313x.2000.00874.x. [DOI] [PubMed] [Google Scholar]
- Maurel C. Plant aquaporins: novel functions and regulation properties. FEBS Letters. 2007;581:2227–2236. doi: 10.1016/j.febslet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N. Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiology. 2004;134:684–693. doi: 10.1104/pp.103.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R. Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. The Plant Cell. 2002;14:727–739. doi: 10.1105/tpc.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. The Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker RH, Elleman CJ, Dickinson HG. Control of pollen hydration in Brassica requires continued protein synthesis, and gylcosylation is necessary for intraspecific incompatibility. Proceedings of the National Academy of Sciences, USA. 1988;85:4340–4344. doi: 10.1073/pnas.85.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Mahlknecht G, Obermeyer G. Measuring the osmotic water permeability of the plant protoplast plasma membrane: implications of the non osmotic volume. Journal of Membrane Biology. 2007;215:111–123. doi: 10.1007/s00232-007-9011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. Water flow in plants and its coupling to other processes: an overview. Methods in Enzymology. 1989;174:183–225. [Google Scholar]
- Temmei Y, Uchida S, Hoshino D, Kanzawa N, Kuwahara M, Sasaki S, Tsuchiya T. Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Letters. 2005;579:4417–4422. doi: 10.1016/j.febslet.2005.06.082. [DOI] [PubMed] [Google Scholar]
- Tiwari SC, Polito VS, Webster BD. In dry peer (Pyrus communis) pollen, membranes assume a tightly packed multilamellate aspect that disappears rapidly upon hydration. Protoplasma. 1990;153:157–168. [Google Scholar]
- Twell D, Yamguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membane aquaporins interact to regulate their subcellular localization. Proccedings of the National Academy of Sciences, USA. 2007;104:12359–12364. doi: 10.1073/pnas.0701180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U. Water relations of plant cells: pressure probe technnique. Methods in Enzymology. 1989;174:338–366. [Google Scholar]
- Zonia L, Cordeiro S, Tupy J, Feijó JA. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3–6-tetrakisphosphate. The Plant Cell. 2002;14:2233–2249. [PubMed] [Google Scholar]
- Zonia L, Müller M, Munnik T. Hydrodynamics and cell volume oscillations in the pollen tube apical region are integral components of the biomechanics of Nicotiana tabacum pollen tube growth. Cell Biochemistry and Biophysics. 2006;46:209–232. doi: 10.1385/CBB:46:3:209. [DOI] [PubMed] [Google Scholar]
- Zonia L, Munnik T. Life under pressure: hydrostatic pressure in cell growth and function. Trends in Plant Science. 2007;12:90–97. doi: 10.1016/j.tplants.2007.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Typical pollen tubes of control and transformed lily pollen are growing in germination medium.