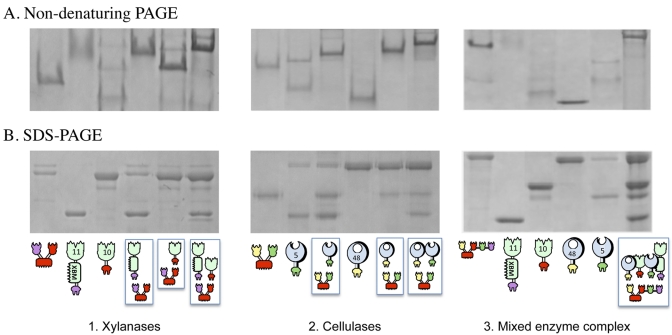
FIG 2 .
Electrophoretic mobility of components and assembled complexes on nondenaturing and denaturing gels. For the two xylanase gels, equimolar concentrations of the chimeric enzymes (11A-xbm-a and 10B-t) and their matching divalent scaffoldin (Scaf·AT) were combined. For the two cellulase gels, equimolar concentrations of the chimeric enzymes (f-5A and b-48A) and their matching divalent scaffoldin (Scaf·BF) were combined. For the two mixed-enzyme complex gels, equimolar concentrations of the chimeric enzymes (f-5A, b-48A, 11A-xbm-a, and 10B-t) and their matching tetravalent scaffoldin (Scaf·BTAF) were combined. The single fusion proteins and the mixtures were subjected to nondenaturing PAGE (A) and denaturing SDS-PAGE (B). Analysis of the matching components by nondenaturing PAGE indicates their near-complete interaction as a single major band formed. The schematic representations below the gels are explained in the symbol key in Fig. 1 and in the legend to Fig. 1.