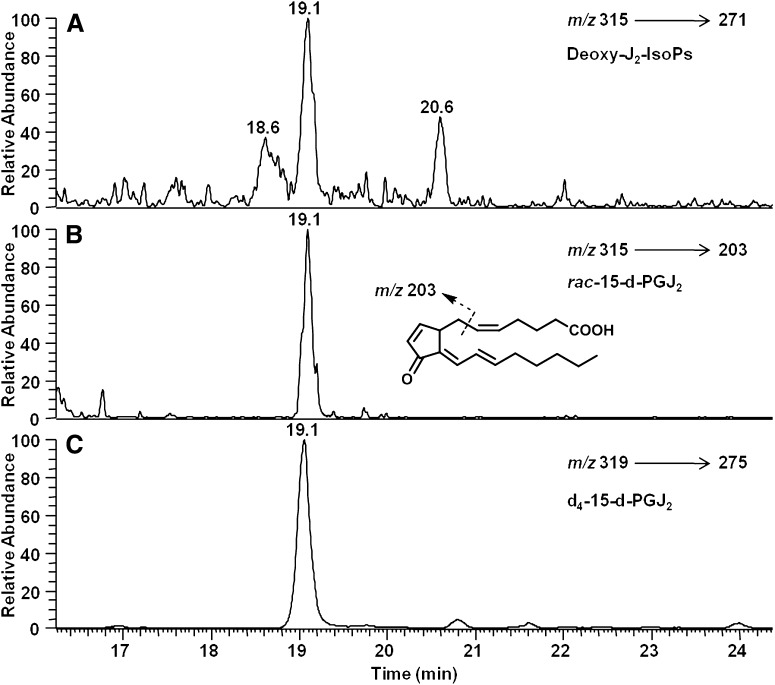
Fig. 9.
Analysis of putative deoxy-J2-IsoPs in vivo by LC/MS/MS using SRM. LC/MS/MS analysis of (A) deoxy-J2-IsoPs regioisomers and (B) the 15-series deoxy-J2-IsoPs (rac-15-d-PGJ2) generated in vivo esterified in liver phospholipids in rats after treatment with CCl4 and (C) chemically synthesized [2H4]15-d-PGJ2 (d4-15-d-PGJ2). The mass spectrometer was operated in the negative ion mode with SRM for the precursor-to-product transitions of m/z 315–271 for all deoxy-J2-IsoPs regioisomers (chromatogram A), m/z 315–203 for rac-15-d-PGJ2 (chromatogram B), and m/z 319–275 for d4-15-d-PGJ2 (chromatogram C).