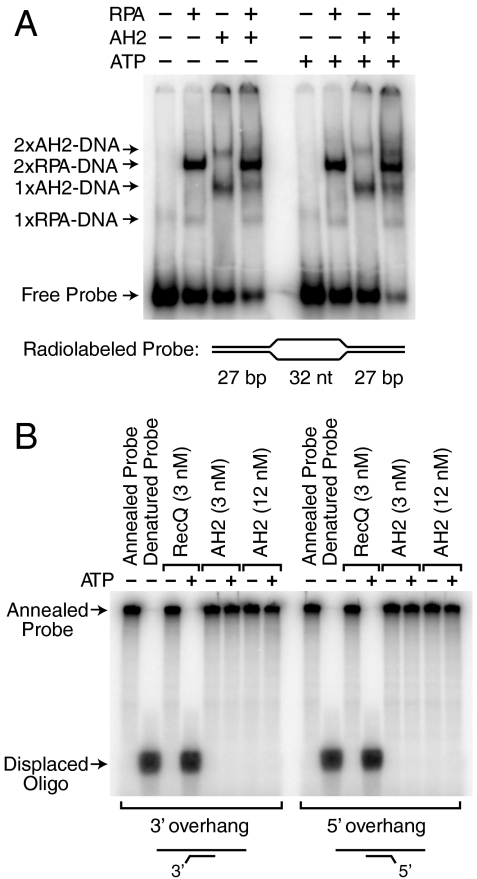
Fig. 4.
AH2 is neither an RPA-removing enzyme nor a conventional helicase. (A) AH2 does not mediate the displacement of RPA from DNA. Gel mobility shift experiments were performed with radiolabeled bubble DNA that contains two high-affinity sites for RPA (the two 32-nt ssDNA segments) and for AH2 (the two DNA forks). A 10-fold molar excess of unlabeled d(CT)30 was added in the binding reactions subsequent to the binding of RPA to the probe DNA. AH2 (3 nM), RPA (3 nM), and ATP (1.5 mM) were included, as indicated. The apparent compositions of the shifted complexes are specified. (B) AH2 does not exhibit helicase activity. Helicase assays were performed with partial duplex DNA substrates, as indicated, containing a radiolabeled oligonucleotide annealed to single-stranded phiX174 DNA. Purified AH2 or RecQ (as a positive control for a helicase) were incubated with the DNA substrates in the absence or presence or ATP. The products were resolved by electrophoresis in a 10% polyacrylamide gel.