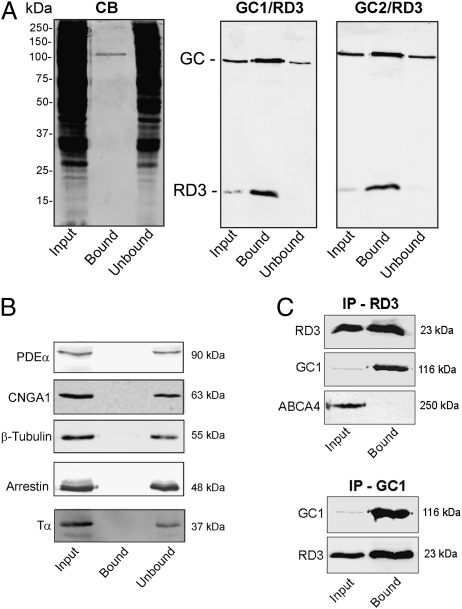
Fig. 2.
Coimmunoprecipitation of GC1 and GC2 with RD3. (A) A detergent-solubilized WT mouse retinal extract was incubated with an Rd3-9D12 immunoaffinity matrix. After the unbound protein was removed, the bound protein was eluted from the matrix with SDS for analysis on Coomassie blue–stained gels (CB) and Western blots labeled with the Rd3-9D12 antibody against RD3 and polyclonal antibodies against GC1 and GC2 (GC1/RD3 and GC2/RD3). Approximately 70 μg of protein was applied to the input (retinal extract) and unbound lanes. (B) The same Western blots as in A, but labeled with antibodies to PDE-α, CNGA1, β-tubulin, arrestin, and transducin (Tα). These proteins did not coimmunoprecipitate with RD3. (C) HEK293T cells coexpressing RD3 and either GC1 or ABCA4 as a control were immunoprecipitated on an Rd3-9D12 immunoaffinity matrix (IP-RD3) or an GC1-2H6 matrix (IP-GC1), and the input and bound fractions were analyzed on Western blots labeled for RD3 (Rd3 9D12 antibody), GC1 (GC-2H6 antibody), or ABCA4 (Rim-3F4 antibody). GC1 coimmunoprecipitated with RD3, but ABCA4 did not.