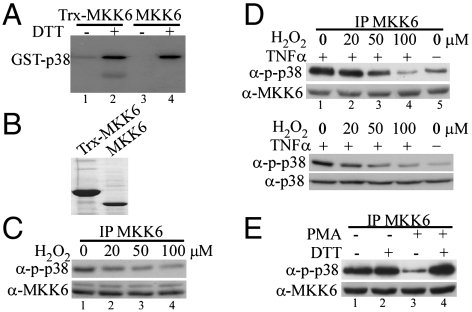
Fig. 1.
The kinase activity of MKK6 was sensitive to oxidation both in vitro and in cells. (A) The bacterially expressed human MKK6 with (lanes 1 and 2) or without (lanes 3 and 4) a Trx tag was purified and extensively dialyzed in the absence of any reducing agent. The purified MKK6 was subjected to in vitro kinase assays using GST-p38(KM) as a substrate in the presence or absence of 1 mM DTT. The phosphorylated p38 was detected by autoradiography. (B) The purified MKK6 with or without a Trx tag was electrophoresed on a SDS-polyacrylamide gel and visualized by Coomassie blue staining. (C and D) HeLa cells were either left untreated or treated with increasing doses of H2O2 as indicated. In C, cells were harvested after 5 min of H2O2 treatment. In D, cells were pretreated with H2O2 for 5 min followed by stimulation with 10 ng/mL TNFα for 10 min. (E) Serum-starved RAW264.7 cells were either left untreated or treated with 1 μM PMA for 30 min. The same amount of the endogenous MKK6 from C–E was immunoprecipitated from whole cell extracts (WCE) and subjected to in vitro kinase assays with GST-p38(KM) as a substrate. The phosphorylated p38 (p-p38) was revealed by Western blot analysis. Ten percent of total WCE used in the immunoprecipitation experiments in C–E was analyzed by Western blotting to reveal the amount of MKK6 as well as p-p38 and total p38 (D) in each sample.