Table 2. Differential equations.
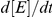
|

|
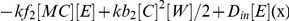
|
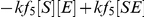
|
||
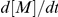
|

|
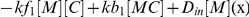
|
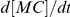
|

|
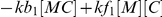
|
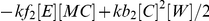
|
||
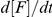
|

|
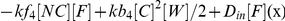
|
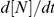
|

|
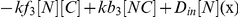
|
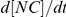
|

|
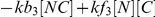
|
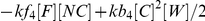
|
||
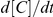
|

|
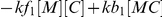
|
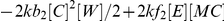
|
||
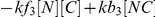
|
||
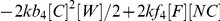
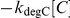
|
||
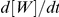
|

|
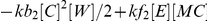
|
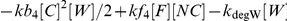
|
||
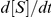
|

|
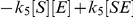
|
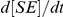
|

|
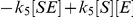
|
Full list of the differential equations that influence the change in chemical concentrations. Includes all chemical pathways used in the experiments. Parameters 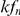 and
and 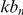 represent the reaction rate constants for the
represent the reaction rate constants for the  th reaction in the forward or backward direction. Those constants with ‘degK’ subscripts represent the rate of degradation of chemical K and constants with ‘
th reaction in the forward or backward direction. Those constants with ‘degK’ subscripts represent the rate of degradation of chemical K and constants with ‘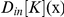 ’ subscripts represent the concentration of resource
’ subscripts represent the concentration of resource 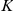 diffusing into the system from the environment at position
diffusing into the system from the environment at position  . Table 1 indicates the values of the constants.
. Table 1 indicates the values of the constants.
