Figure 8.
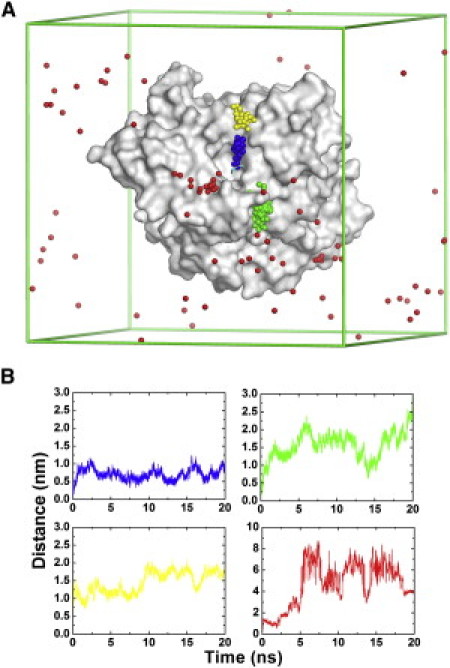
MD trajectories for movement of TChP in native and mutated TcAChE in which acidic residues had been neutralized. (A) The movement of TChP as extracted, at 200-ps intervals, from four MD trajectories, is shown, with the individual balls representing the nitrogen of TChP. The blue trajectory is that of one of the forty trajectories in simulation group III. The simulation conditions in the other three trajectories were similar to those for the blue trajectory, except that the charges of side-chain carboxyl oxygen atoms were set to zero for D285 (green trajectory), for D72, E73, E273, E276, and D285 (yellow trajectory), and for all aspartate and glutamate residues (red trajectory). The molecular surface of the protein (PDB code 2c5g) is displayed in light gray, and the lines of the simulation box are depicted in green. (B) The distance of TChP from its binding site at the PAS in the four trajectories displayed in Fig. 8A, with the same color coding (blue, green, yellow, and red).
