FIG 1 .
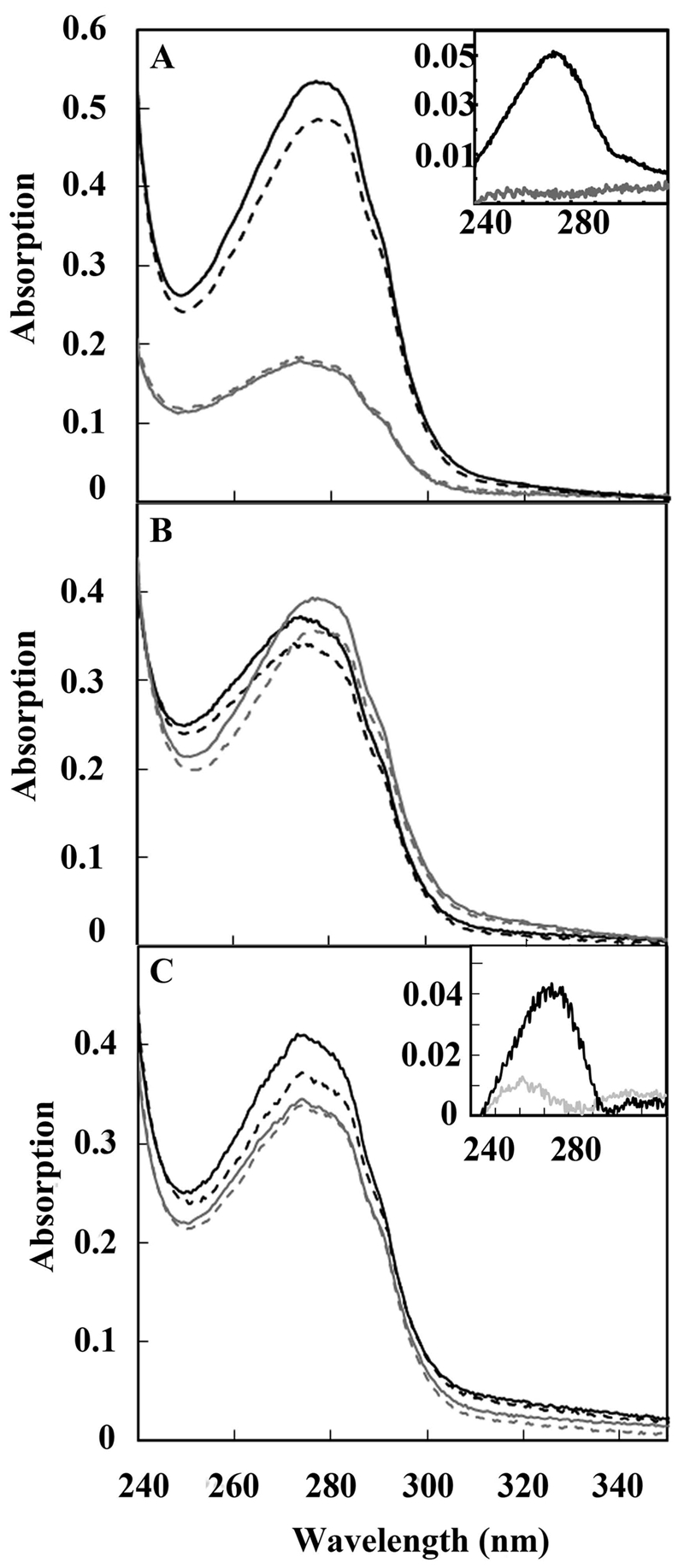
UV spectra of ubiquinone bound to RegB. (A) Spectrum of isolated oxidized full-length wild-type RegB (solid black line) containing 0.82 mol ubiquinone/mol protein that exhibits a decrease in absorbance at 275 nm upon reduction with NaBH4 (dashed black line). A control of truncated RegB″ C265S, which lacks the membrane-spanning domain, shows no reduction in intensity upon addition of NaBH4, thereby indicating the lack of ubiquinone (gray lines). The inset shows a difference spectrum before minus after reduction with NaBH4 of full-length wild-type RegB (black line) and of RegB″ C265S (gray line). (B) A red shift of maximum absorption of ubiquinone caused by an increasing amount of bound ubiquinone. Different batches of purified RegB C265S were scanned before (solid lines) and after (dashed lines) NaBH4 reduction. Batch A (black lines) contains 0.44 mol ubiquinone/mol RegB C265S, while batch B (gray lines) contains 0.62 mol ubiquinone/mol RegB C265S. (C) Isolated RegB C265S was reduced and ultrafiltered in buffer containing NaBH4 as described in the text, and then the sample was divided. The gray scans are of the portion that was scanned before (solid gray line) and after (dashed gray line) reduction with NaBH4. The absence of oxidized ubiquinone in this fraction is evident from the difference scan (inset gray line) of before minus after addition of reductant, which shows only a small peak. The black lines are of the other portion, which was reoxidized by the addition of the oxidant K3Fe(CN)6 at 22°C for 5 min (solid black line), followed by the addition of the reductant NaBH4 (dashed black line). The retention of ubiquinone is evident from the difference spectrum (inset black line) of this preparation before minus after reduction with NaBH4.
