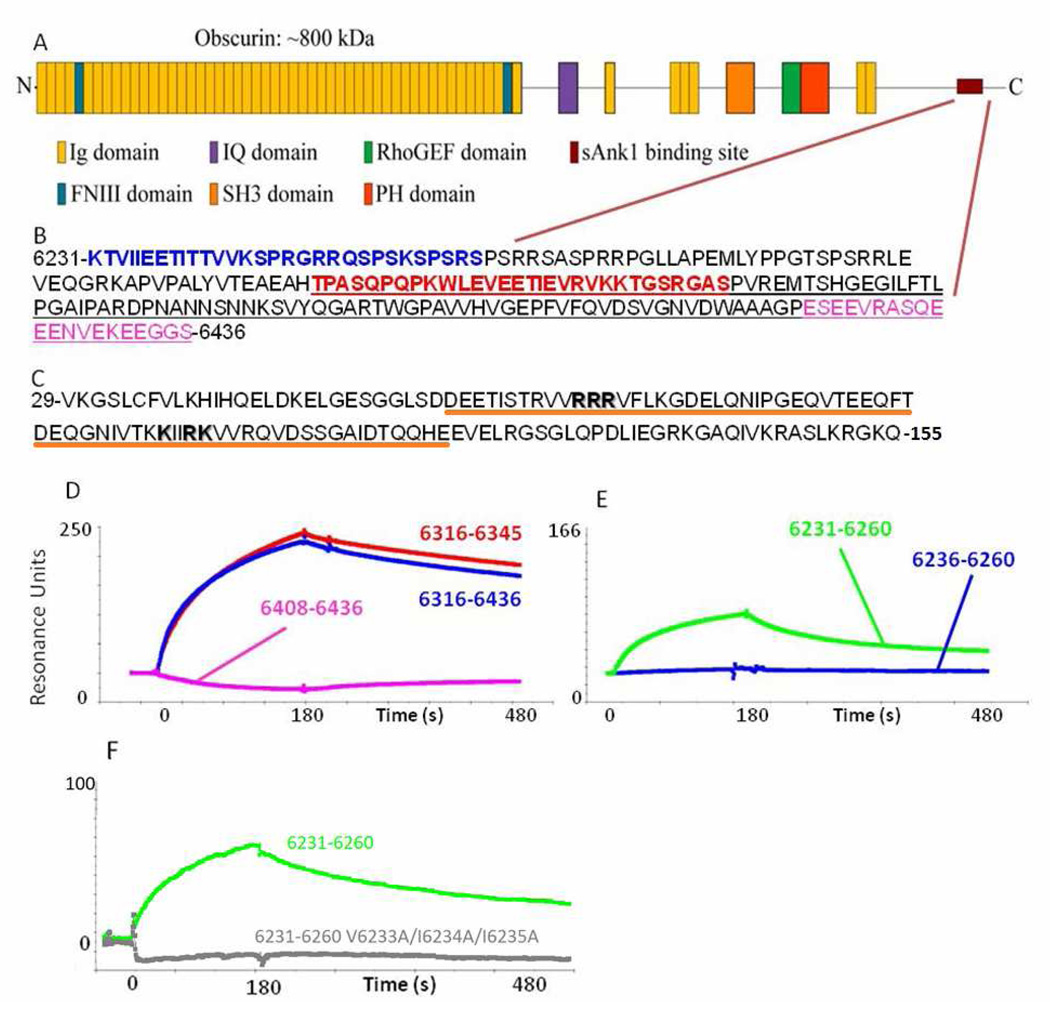
Fig. 1. The sequences of obscurin and sAnk1 that mediate binding.
(A) Cartoon of the domain organization of obscurin A. The burgundy lines indicate the region of the molecule shown in B. (B) The sequence of residues corresponding to amino acids 6231–6436 of obscurin A (Rattus norveigicus). Blue text denotes the sequence identified by Bagnato et al.6. The red sequence denotes the residues identified as the minimal binding domain and used for experiments in this manuscript (see D and E). The underlined sequence is that identified by Kontrogianni-Konstantopoulos et al.12. The sequence in pink shows a sequence rich in electronegative amino acids that is neither necessary nor sufficient to bind sAnk1 (see D, pink). (C) Sequence of residues 29–155 of sAnk1 of the rat. Residues shown by Borzok et al. to mediate binding to obscurin are shown in bold; residues 57–122, underlined in orange have previously been modeled as ankyrin-like repeats13. (D, E) Surface plasmon resonance assays of binding of fusion constructs of sAnk129–155 to different sequences of obscurin. (D) Obsc6316–6345 (red), Obsc6316–6436 (blue), Obsc6408–6436 (pink). (E) Obsc6231–6260 (green), Obsc6236–6260 (blue). (F) Obsc6231–6260 (green), Obsc6231–6260 V6233A/I6234A/I6235A (grey).