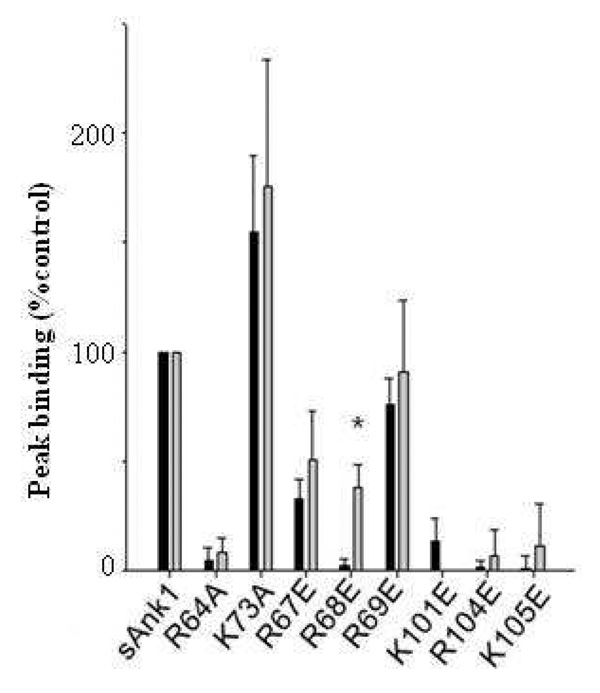
Fig. 4. Site-directed mutants of sAnk1 reduce binding of Obsc6231–6260 and Obsc6316–6345 to a similar extent.
We assayed the effects on binding of mutating the lysine or arginine residues of sAnk1 involved in binding obscurin (see Fig. 1) to glutamates or alanines. Grey bars: Binding to Obsc6231–6260 (normalized to maximal binding, measured with WT sAnk1); black bars: binding to Obsc6316–6345 (normalized similarly). With the exception of sAnk1 R68E, mutations in sAnk1 have similar effects on binding to each of the binding sites on obscurin. n=5 for all experiments. Error bars, S.D.; * indicates a significant difference (p < .05).