Figure 3.
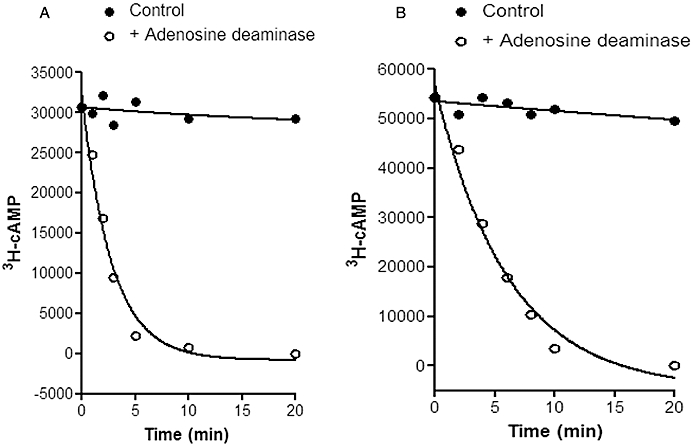
Effect of the phosphodiesterase (PDE) inhibitor rolipram on 3H-cAMP breakdown and steady-state levels of cAMP in 3H-adenine-labelled brain slices from guinea-pig cerebral cortical slices. Data are taken from Donaldson et al. (1988a). In (A) brain slices were pre-incubated with 0.1 mM adenosine for 10 min to achieve a steady-state level. At time zero adenosine deaminase was added (1.2 U·mL−1) to rapidly remove adenosine and cAMP levels then rapidly fell under the influence of endogenous PDEs. In (B) the same experiment was undertaken in the presence of the PDE inhibitor rolipram (0.1 mM). In this case a new and much higher steady-state level of cAMP was achieved. This is because the reduced PDE activity caused by rolipram allows the cAMP levels to rise until they achieve a new equilibrium at which cAMP synthesis is matched again by cAMP metabolism. The continued ability to metabolize cAMP under these conditions is clearly evident when adenosine deaminase is once again applied (B). The influence of the competitive PDE inhibitor rolipram is evident, however, in the reduced rate at which cAMP levels fall in (B). In both (A) and (B) the solid symbols show that the steady-state level of cAMP is well maintained in the absence of adenosine deaminase.
