Figure 5.
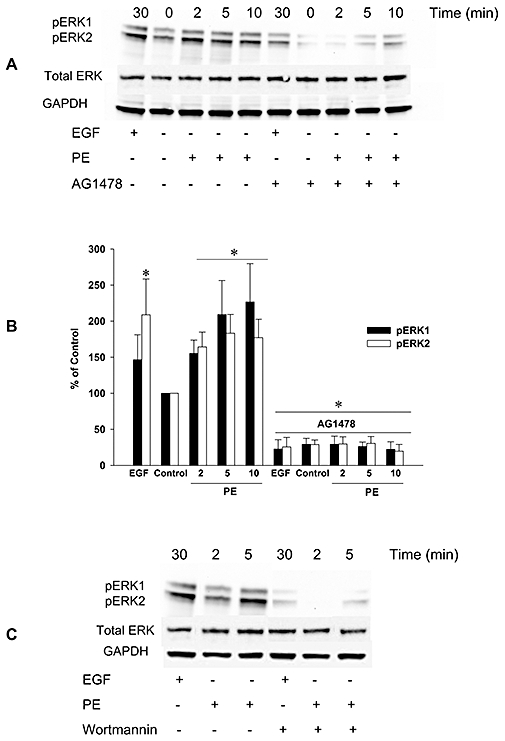
Involvement of EGFR in α1-adrenoceptor mediated stimulation of phosphorylation of ERK1/2 in endothelium-denuded rat thoracic aorta segments. Phosphorylation of ERK1/2 (pERK1, pERK2) after stimulation with epidermal growth factor (EGF; 30 min) or phenylephrine (PE; 2, 5, and 10 min) was determined by immunoblotting (A). The results from all of the experiments shown are representative of four independent experiments in isolated endothelium-denuded thoracic aorta segments from different rats. Phosphorylation of ERK1/2 was normalized to GAPDH, quantified as the percentage of control (PE, 0 min) and expressed as mean ± SEM (B). *P < 0.05 versus Control (Mann–Whitney U-test). EGFR tyrosine kinase inhibitor, AG1478 (A) and PI3K inhibitor, wortmannin (C) blocked the phosphorylation of ERK1/2 after stimulation with EGF or phenylephrine. EGFR, epidermal growth factor receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pERK, phosphorylated extracellular signal-regulated kinases.
