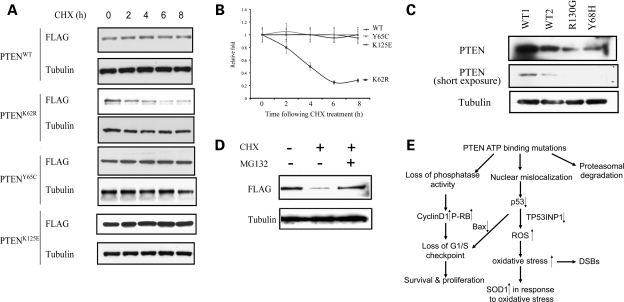
Figure 5.
PTEN ATP-binding mutations reduce protein stability. (A) CHX-chase analyses of PTEN degradation in MCF-7 cells overexpressing WT or mutant PTEN. Cells were incubated with CHX (50 µg/ml) and were harvested at the indicated time points. Whole protein lysates were harvested and ran for western blots using anti-FLAG antibody for transfectant PTEN and anti-tubulin antibody as a loading control. (B) Protein half-lives of PTENWT, PTENK62R, PTENY65C and PTENK125E. Linear plots represent the averages range of two independent experiments. (C) Western blots of whole cell lysates derived from lyphoblastoid cells isolated from two CS patients with indicated genotypes (PTENY68H/WT and PTENR130G/WT) as well as two normal WT controls. (D) Proteasomal degradation of PTENK62R. PTEN null BT-549 cells were transfected with FLAG-tagged PTENK62R and were treated with either DMSO, CHX (50 µg/ml) or a combination of CHX and MG132 (10 µm) for 16 h. Exogenously expressed PTEN protein levels were determined by western blot using an anti-FLAG antibody. (E) Model for tumorigenesis induced by PTEN ATP-binding mutations. Mutations within PTEN ATP-binding motifs lead to PTEN nuclear mislocalization and decreased p53 level through an MDM2-independent way. Impaired activities of PTEN and p53will increase the vulnerability of cells to oxidative stress, as presented by enhanced ROS, upregulated SOD1 and DNA damage. Loss of phosphatase activities in the PTEN ATP-binding mutants, together with p53 deactivation, can lead to the loss of G1/S checkpoint, as presented with abnormal cell proliferation and survival. For the mutant such as K62R, it undergoes proteasomal degradation. All these quantitative and qualitative impairments compromise PTEN's tumor-suppressor activity.