Table 1.
Coupling of aryl halides with TIPS-SH catalyzed by Pd(OAc)2 and CyPF-tBu ligand.[a]
 | ||||
|---|---|---|---|---|
| Entry | ArX | Cat. [mol%] | Product | Yield [%] |
| 1 | 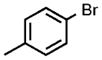 |
0.05 | 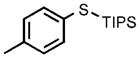 |
98 |
| 2[b] | 0.05 | 88 | ||
| 3[c] | 0.05 | 90 | ||
| 4[d] | 0.1 | 91 | ||
| 5[e] | 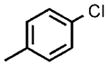 |
0.1 | 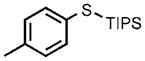 |
96 |
| 6 | 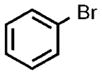 |
0.05 | 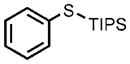 |
91 |
| 7 | 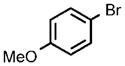 |
0.25 | 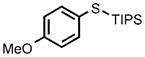 |
96 |
| 8 | 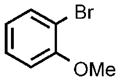 |
0.25 | 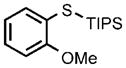 |
99 |
| 9 | 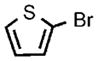 |
0.25 | 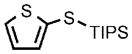 |
76 |
| 10 | 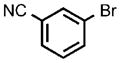 |
0.25 | 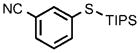 |
87 |
| 11 | 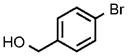 |
0.25 | 74 | |
| 12 | 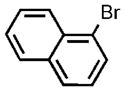 |
0.05 | 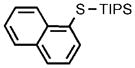 |
97 |
| 13 | 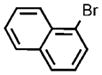 |
0.05 | 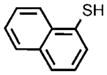 |
95[f] |
Reactions were conducted with a 1:1 ratio of metal to ligand, 1 mmol of both ArX and thiol, and 1.1 equiv of LiHMDS at 110°C in toluene (1.5 mL) requiring 2–4 h to complete.
Reaction performed with NaOtBu as base.
Reaction conducted in DME.
Reaction performed at 90°C.
Reaction required 12 h to complete.
TBAF (2 equiv) was added to the crude mixture and stirred 30 min at RT.
