FIGURE 5.
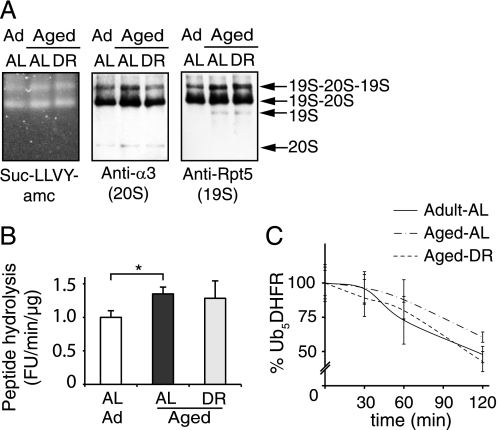
26S proteasomes isolated from aged and adult muscles show similar size distributions and similar abilities to hydrolyze peptide substrates and ubiquitin conjugates. A, gastrocnemius muscles from individual animals were pooled (60 mg each; n = 7) and 26S proteasomes were isolated using the UBL affinity technique (7). Purified proteasomes were analyzed on native PAGE by peptidase activity (Suc-LLVY-AMC overlay assay) and immunoblot. B, the specific activity of purified 26S proteasomes was determined using Suc-LLVY-AMC and expressed relative to the activity of adult proteasomes. The averaged results from three different purifications are shown. C, to assay the degradation of a ubiquitylated protein, 50 nm Ub5DHFR was incubated with 2 nm 26S for 0, 30, 60, and 120 min. The amount of Ub5DHFR was determined by immunoblot and quantified by densitometry. The panel presents averages of two independent measurements, each analyzed by two sets of immunoblots. Error bars are SD. *, p < 0.05.