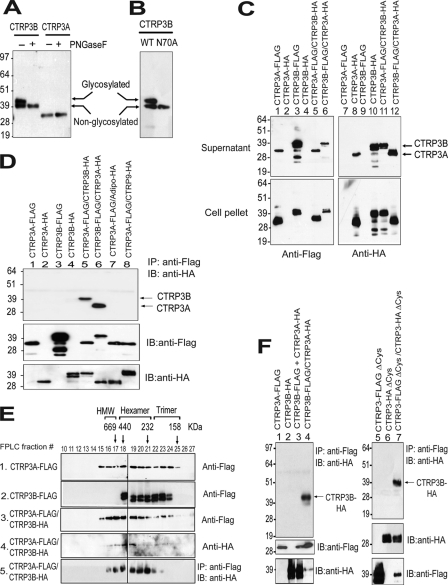
FIGURE 6.
Human CTRP3A forms hetero-oligomers with CTRP3B, protecting CTRP3B from proteolytic cleavage. A, immunoblot analysis was carried out on conditioned media containing FLAG-tagged CTRP3A or CTRP3B incubated with (+) or without (−) peptide:N-glycosidase F (PNGaseF) to determine the presence of N-linked glycans. B, conditioned media containing FLAG-tagged wild-type (WT) and mutant (N70A) CTRP3B were subjected to immunoblot analysis. C, supernatant and cell lysate containing epitope-tagged CTRP3A and/or CTRP3B were subjected to immunoblot analysis. D, secreted epitope-tagged CTRP3A and/or CTRP3B were immunoprecipitated (IP) with the anti-FLAG affinity gel and immunoblotted (IB) with the anti-HA antibody. E, size exclusion chromatographic (FPLC) analysis of secreted epitope-tagged CTRP3A and/or CTRP3B. Fractions 10–27 were subjected to immunoblot analysis (panels 1–4). Additionally, fractions 10–27 were immunoprecipitated with the anti-FLAG affinity gel and immunoblotted with the anti-HA antibody (panel 5). The indicated molecular weight markers (669, 440, 232, and 158 kDa) correspond to the peak elution fraction of molecular standard thyroglobulin, ferritin, catalase, and aldolase, respectively. FPLC fractions that correspond to adiponectin trimers, hexamers, and high molecular weight oligomers are indicated. F, co-expressed proteins (lane 4) or a mixture of separately expressed protein (lane 3) were immunoprecipitated with the anti-FLAG affinity gel and immunoblotted with the anti-HA antibody (top). Replicate blots were probed for the presence of epitope-tagged input proteins. The cysteine mutant of CTRP3A and/or CTRP3B (lanes 5–7) were immunoprecipitated with the anti-FLAG affinity gel and immunoblotted with the anti-HA antibody (top). Replicate blots were probed for the presence of epitope-tagged input proteins.