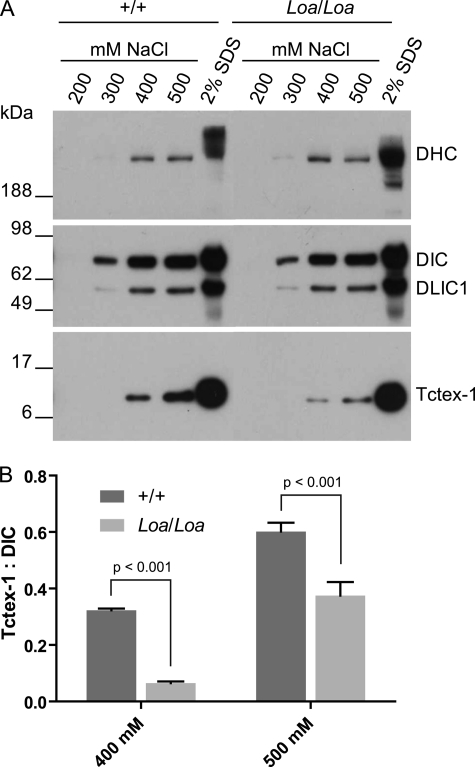
FIGURE 4.
Dynein light chain Tctex-1 is more tightly associated with DICs in Loa/Loa. A, dynein complex was immunoprecipitated from cytosolic extracts isolated from newborn mouse brains using anti-DIC antibody. Proteins were sequentially eluted from the Sepharose 4B beads by stepwise increases in NaCl concentration from 200 to 500 mm, followed by a final extraction with 2% SDS-PAGE loading buffer. Equal volumes of the eluates were loaded on to 4–12% gradient SDS-polyacrylamide gels followed by electrophoresis and immunoblotting to detect DHC, DIC, DLIC1, and Tctex-1. The results revealed that the dynein complex is stable in 200 mm NaCl, and only a small part of dynein polypeptides was released from the beads in 300 mm NaCl. However, much more Tctex-1 was released from the beads at 400 and 500 mm NaCl in +/+ than in Loa/Loa. B, Tctex-1/DIC ratio of Loa/Loa was compared with that of +/+ by densitometry quantification of Tctex-1 and DIC bands. The Tctex-1/DIC ratios are significantly different between Loa/Loa and +/+ at 400 mm NaCl (p < 0.001) and 500 mm NaCl (p < 0.001), n = 4.