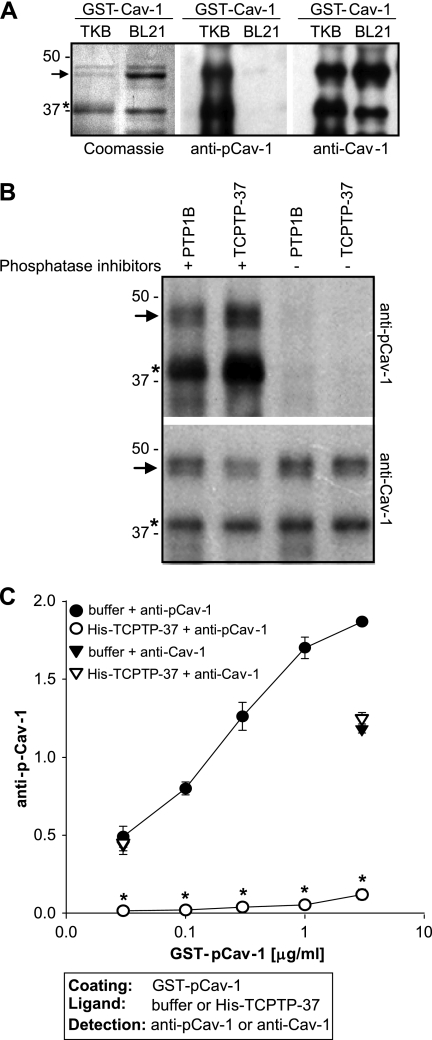
FIGURE 5.
Constitutively active TCPTP dephosphorylates caveolin-1. A, Coomassie staining of purified GST-pCav-1 and GST-Cav-1 (2 μg/lane). 20 ng of purified GST-pCav-1 and GST-Cav-1 were separated in 12% SDS-PAGE, transferred to nitrocellulose, and blotted with anti-Tyr(P)14-pCav-1 or anti-Cav-1 antibodies. The arrow indicates full-length constructs, whereas the asterisk indicates a cleaved product still retaining the Cav-1 phosphorylation site (Tyr14). B, GST-pCav-1 (0.4 μg/ml) was incubated with constitutively active TCPTP (TC-37, 0.4 μg/ml) or PTP1B (0.4 μg/ml) with or without tyrosine phosphatase inhibitors. 30 min later, the samples were analyzed by Western blot for levels of phosphorylated and total Cav-1. C, GST-pCav-1, coated at the concentrations indicated, was incubated with either phosphatase buffer or TCPTP-37 followed by incubation with anti-phosphorylated or anti-Cav-1 antibodies. One representative experiment performed in triplicate is shown. Two experiments were performed with similar results. *, indicates significant differences (p < 0.05) between wells coated with pCav-1 incubated with or without His-TCPTP-37. Error bars, S.D.