Fig. 2.
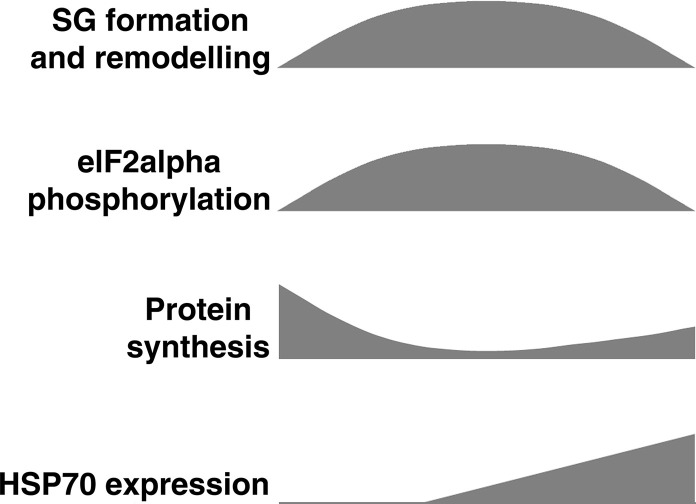
Comparative time-course of SG formation, eIF2alpha phosphorylation, protein synthesis, PB induction and heat shock protein expression upon stress induction. Maximal SG formation, eIF2alpha phosphorylation and protein synthesis inhibition occur quite simultaneously, between 1 and 2 h upon oxidative stress in mammalian or insect cells, or around 2–4 h upon ER-stress, respectively [16], [17]. All trough during the response, SG grow, undergo fusion and fission and remodelate. They can incorporate or lose components during the response (see text). Two hours after oxidative stress induction, the foci begin to dissolve synchronously and they completely vanish 1 h later. A similar time-course, with the time of maximal SG formation at around 2 h and a slower dissolution phase is observed upon ER-stress induction. SG dissolution occurs with similar time-course either in the presence or absence of oxidative or ER-stress inductors, or upon booster applications [16], [17]. SGs are induced rapidly by inhibitors of translation initiation, and do not dissolve unless the drug is removed (Loschi and Boccaccio unpublished). eIF2alpha phosphorylation reaches maximal levels and may go back down basal levels during SG dissolution. Protein synthesis shuts off at the time of maximal SG formation and then partially recovers during SG disassembly. This correlates with HSP70 expression, which keeps accumulating beyond SG disassembly. Synthesis of heat shock proteins lasts for several hours, whereas recovery of normal protein synthesis takes a longer time. PBs are induced by cellular stress, then they may return to basal conditions, move to the perinucleus or vanish, and their components can be incorporated to SGs [16], [50], [122]. Paralleling SG formation in the cytoplasm, the formation of Nuclear Stress Bodies (nSBs) occurs at specific foci in the nucleus (see text). Like SGs, nSBs are transient and remodellate during the response.