Figure 1. Multi-scale Systems Biology of the VEGF ligand-receptor system.
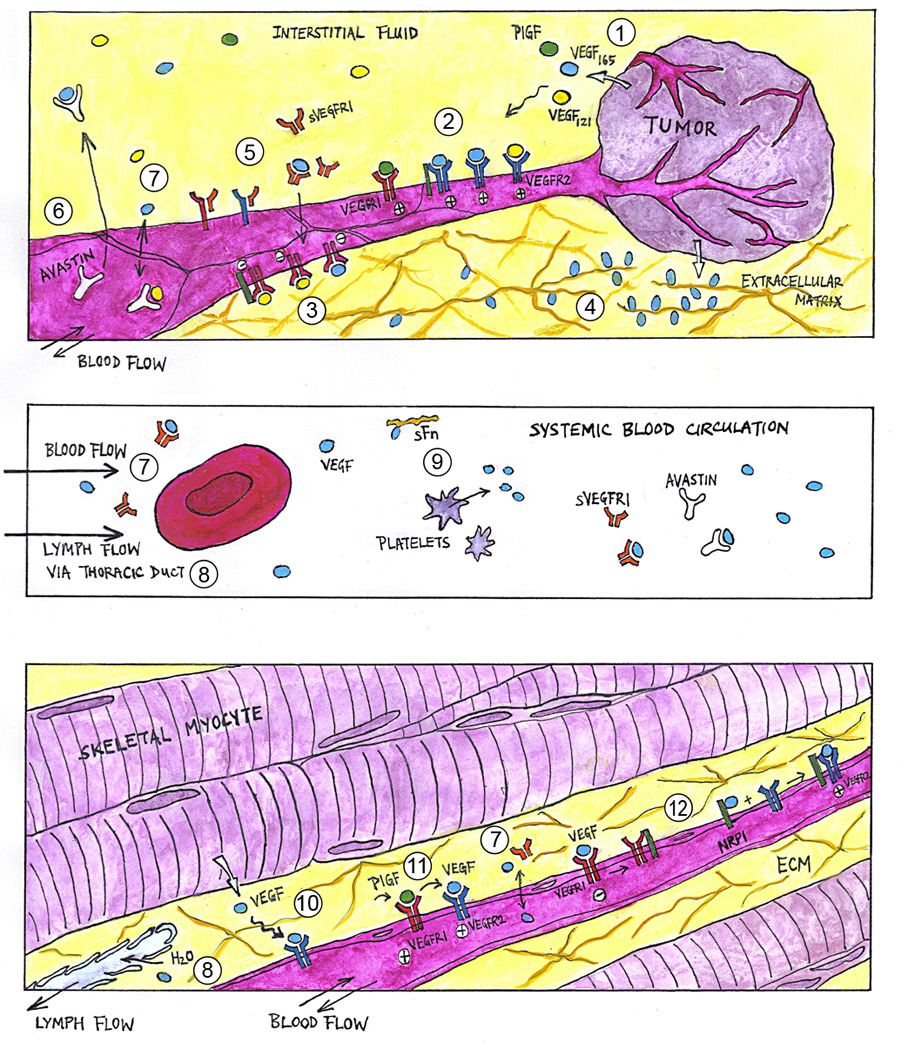
(1) Hypoxia, such as that in growing tumor tissues (top panel), trigger the expression and extracellular secretion of VEGF ligand proteins, e.g., VEGF-A (isoforms VEGF121 and VEGF165) and PlGF. At the cellular level, VEGF ligands diffuse towards nearby capillary surfaces, binding endothelial cell surface receptors (VEGFR1, VEGFR2) and co-receptors (NRP1) in various configurations to activate (2) pro-angiogenic and (3) anti-angiogenic downstream signaling. (4) At the tissue level, VEGF ligands with heparin-binding domains can be sequestered at heparan sulfate proteoglycan sites in the extracellular matrix (ECM), forming chemotactic gradients that guide capillary sprout migration. (5) Soluble VEGFR1 (sVEGFR1) potentially modulates angiogenic signaling via ligand trapping or dominant-negative heterodimerization with transmembrane VEGFR monomers. (6) Humanized anti-VEGF antibodies (e.g., Avastin®), through their capacity to sequester specific VEGF ligands, are being investigated as anti-angiogenic agents in cancer treatment. At the whole-organism level, macromolecules such as VEGF ligands and their soluble receptors may have systemic effects, as they enter the blood circulatory system (middle panel) through inter-tissue transport processes including (7) transcapillary vascular permeability and (8) lymphatic drainage of the interstitial fluid. (9) Other VEGF carriers in the blood include soluble fibronectin and platelets. (10) Similarly in skeletal muscle (bottom panel), VEGF ligand expression is upregulated in hypoxic myocytes. However in peripheral arterial disease, the angiogenic response is insufficient to alleviate muscle ischemia. Pro-angiogenic therapies under investigation include VEGF-A delivery through cell, gene, and protein therapy. Adjuvant therapeutic targets include (11) PlGF (thought to work synergistically through VEGFR1 signaling or ligand shifting) and (12) NRP1 (via presentation of VEGF to VEGFR2 or reducing anti-angiogenic VEGF·VEGFR1 complexes).
