Abstract
Several substances, including glutamine and propionic acid but in particular butyric acid, have been proposed to be important for colonic health. β-Glucans lead to the formation of comparatively high amounts of butyric acid, and germinated barley foodstuff obtained from brewer’s spent grain (BSG), containing high amounts of β-glucans and glutamine, has been reported to reduce the inflammatory response in the colon of patients with ulcerative colitis. The present study examines how 3 barley products, whole grain barley, malt, and BSG, affect SCFA in the hindgut and serum of rats and whether the addition of Lactobacillus rhamnosus 271 to each of these diets would have further effects. Amino acids in plasma and the cecal composition of the microbiota were also analyzed. The butyric acid concentration in the distal colon and serum was higher in the malt groups than in the other groups as was the serum concentration of propionic acid. The concentrations of propionic and butyric acids were higher in the cecum and serum of rats given L. rhamnosus than in those not given this strain. The proportion of plasma glutamine and the cecal number of bifidobacteria were lower in the malt groups than in the other groups. L. rhamnosus decreased the number of cecal bifidobacteria, whereas plasma glutamine was unaffected. We conclude that malt together with L. rhamnosus 271 had greater effects on propionic and butyric acid concentrations in rats than the other barley products. This is interesting when developing food with effects on colonic health.
Introduction
Butyric acid, one of the main products of the fermentation of dietary fiber (1), and glutamine are important substrates for the epithelial cells of the colon and may therefore offer protection against colonic diseases. Furthermore, high concentrations of glutamine in the circulatory system have been found to improve immune defense (2, 3). The presence of high amounts of butyric acid in colon may reduce the need of the epithelial cells for glutamine, resulting in an increase of the glutamine concentration in the blood (4). From this point of view, barley is an interesting product, because it may contribute both with glutamine and butyric acid in the colon (5, 6).
Colonic fermentation of barley β-glucans has been shown to give high amounts of butyric acid, whereas germinated barley foodstuff (GBF)4 has been reported to contain high amounts of glutamine (7, 8). Glutamine is bound to the dietary fiber and must reach the colon before it can be liberated to provide a substrate for the colonic mucosa (8). GBF was shown to ameliorate symptoms and inflammation in humans with ulcerative colitis (6) and also in rats in which the condition was induced with dextran sulfate sodium (DSS) (9, 10).
GBF is obtained from brewer’s spent grain (BSG), which is a by-product of the brewing process, by separating the aleurone and scutellum from BSG by milling and sieving (8). In the first step, when brewing beer, whole grain barley (WGB) is germinated and then roasted to obtain malt (11). The malt is then crushed, mixed with water, heated, and filtered. The filtrate is used in the next step, while the precipitated BSG is a by-product often used as cattle feed (8).
SCFA are the principal products formed from colonic fermentation of dietary fiber and other indigestible carbohydrates. The amount of SCFA formed is determined by a number of factors, including substrate availability, transit time, and the number and types of microbiota present in the colon. Lactobacillus rhamnosus is one of the most common bacteria in the bowels of healthy individuals (12) and it is the most extensively used probiotic in clinical trials (13). L. rhamnosus has been found to reduce the mucosal permeability associated with DSS-induced colitis in rats (14) and it has therefore been suggested that it would be useful in the treatment of gastrointestinal diseases such as inflammatory bowel diseases. Furthermore, high amounts of probiotic bacteria may prevent the proliferation of pathogenic bacteria.
The aim of this study was to investigate which barley product led to the highest concentrations of butyric acid and glutamine in the hindgut and blood of rats and whether this was affected by probiotic feeding. For this purpose, 3 barley fractions, WGB, malt, and BSG, found in the production line for beer were chosen. In malt, the glutamine synthesized during germination is enriched compared with WGB, and in BSG the concentration of both β-glucans and glutamine is enhanced (6, 9). The probiotic strain used was L. rhamnosus 271. The hindgut formation of carboxylic acids (CA), the concentrations of SCFA and amino acids in portal blood, and the cecal composition of the bacterial flora (lactobacilli, bifidobacteria, Enterobacteriaceae) were analyzed in healthy rats.
Materials and Methods
Composition of the barley products
The starting material was WGB (Viking Malt). One batch of the grain was used to produce malt and BSG by the brewer Carlsberg Sverige. The 3 barley products were milled to a particle size < 0.5 mm before being included in the various diets, alone or in combination with the freeze-dried probiotic strain, L. rhamnosus 271 (Probi). The nutrient composition of the 3 barley products is given in Table 1.
TABLE 1.
Composition of the WGB, malt, and BSG1
| WGB | Malt | BSG | |
| g/100 g dry weight | |||
| Protein | 9.7 ± 0.2 | 9.8 ± 0.1 | 23.8 ± 0.4 |
| Fat | 3.4 ± 0.0 | 3.6 ± 0.0 | 8.4 ± 0.4 |
| Starch | 62.6 ± 1.3 | 65.7 ± 1.9 | 5.5 ± 0.1 |
| Dietary fiber | 15.4 ± 0.9 | 12.1 ± 0.4 | 58.2 ± 1.6 |
Values are means ± SEM, = 2–3, independent measurements.
Diets
The barley products were added at a concentration of 80 g dietary fiber/kg dry diet. Wheat starch was used to adjust the dry matter content, because this type of starch has previously been shown to be completely digested and therefore does not form any CA (15). The probiotic strain was included daily in the diet at feeding time for each rat (2 × 108 CFU/d). The compositions of the 6 test diets are given in the Supplemental Table 1.
Rats and study design
Male Wistar rats (initial weight, 134 ± 1 g) were obtained from Scanbur. They were randomly divided into 6 groups, 7 rats in each, except in the group fed malt with L. rhamnosus (in which 1 rat died at the start of the experimental period), and were housed individually in metabolic cages (16) in a room maintained at 22°C with a 12-h-light/-dark cycle. Feed intake was restricted to 12 g dry weight/d and the rats were given free access to water. Each group of rats was fed 1 of the 6 experimental diets: WGB, malt, and BSG alone or in combination with L. rhamnosus 271 (Supplemental Table 1).
The rats were allowed to adapt to the diet for 7 d and were then followed for a 5-d experimental period, during which feces and feed residues were collected daily. The fecal samples were stored at –20°C and then freeze-dried and milled before analysis of dietary fiber content. At the end of the experiment, the rats were anesthetized by subcutaneous injection of a mixture of Hypnorm (Division of Janssen-Cilag), Dormicum (F. Hoffman-La Roche), and sterile water (1:1:2) at a dose of 0.15 mL/100 g body weight, after which the cecum, colon (divided into 2 equal parts), and blood were collected. The blood samples were drawn from the portal vein into 2 tubes: 1 for plasma, containing EDTA, and 1 for serum. The tubes containing the plasma and serum samples were centrifuged for 15 min at 2500 × g and then stored at –40°C until the analysis of amino acids and SCFA, respectively. The cecum was weighed with and without its contents, and the pH of the cecal contents was measured. The cecal content was divided between 2 sterile tubes. One was frozen and stored at –40°C pending analysis of the CA. The other contained freezing medium and was immediately frozen in liquid nitrogen and stored at –80°C until analysis of the microbiota. The content of the proximal and distal colon was also frozen and stored at –40°C pending analysis of the CA. The rat experiment was approved by the Ethics Committee for Animal Studies at Lund University (no. M96–07).
Analyses
Barley products.
The protein content was determined with the nitrogen combustion method (17), total starch by the method described in (18), and crude fat by the Schmid-Bondzynski-Ratzlaff method, which is based on hydrolysis in 7.7 mol/L HCl and ethanol for 1 h at 75°C followed by extraction in diethyl ether and petroleum ether (40–60°C, 1:1).
A gravimetric method (19) was used to determine the amount of soluble and insoluble dietary fiber in the barley products. The composition of the soluble and insoluble fiber was analyzed using GLC of the neutral sugars as their alditol acetates, and the content of uronic acids was analyzed with a spectrophotometric method (20). The content of dietary fiber in feces was analyzed in the same way as in the barley products but without the gravimetric step.
CA in hindgut material.
A newly developed GLC method, based on acidified water extraction and direct injection, was used to analyze the SCFA (acetic, propionic, isobutyric, butyric, isovaleric, valeric, caproic, and heptanoic acids) in cecal and proximal and distal colonic contents (21). This method could not be applied to determine succinic and lactic acids due to the strong interactions of these acids with the liquid phase of the column used. These acids were therefore spectrophotometrically quantified with commercially available enzymatic kits according to the manufacturer’s instructions (catalog nos. 10176281035 and 1112821, respectively; Boehringer Mannheim). The sum of the SCFA and succinic and lactic acids is referred to as CA below.
SCFA in serum.
The SCFA in serum were preconcentrated and cleaned up by liquid hollow-fiber extraction followed by GLC according to Zhao et al. (22).
Amino acids.
Amino acids in the barley products and plasma were determined according to (23, 24), respectively. During the hydrolysis of the raw materials, tryptophan was lost and glutamine and asparagine were converted to glutamic and aspartic acid, respectively. Sulfosalicylic acid was added to plasma as a precipitating agent to separate free amino acids from high-molecular weight proteins (25).
Intestinal microbiota.
The cecal samples were thawed and after homogenization, conventional serial dilution was performed. Viable counts and the number of lactobacilli, bifidobacteria, and Enterobacteriaceae were analyzed by conventional microbial techniques according to (26).
Calculations and statistical analyses
The concentration of each CA (μmol/g) was multiplied by the weight of the cecal content to obtain the cecal pool (μmol). The cecal pool values were extrapolated to the complete intake of dietary fiber (4.8 g) to correct for the small amounts of feed residues. The increase in body weight during the experimental period was calculated per gram feed consumed. Bulking capacity was calculated as the amount of fecal dry weight in g/g dietary fiber consumed.
Two-way ANOVA was used to determine the effects of barley products (Fiber), L. rhamnosus 271 (Lr), and their interaction (Fiber×Lr). If the main effect of barley products was significant, the means of the 2 groups fed each barley product were compared using Tukey’s multiple range test (P < 0.05). One-way ANOVA followed by Tukey’s procedure was used to assess which barley product was affected by L. rhamnosus if the interaction was significant (P < 0.05). When error variances were heterogeneous, data were transformed using Box-Cox transformation before ANOVA. Values are presented as means ± SEM and differences resulting in P-values < 0.05 were considered significant. All evaluations were performed with Minitab statistical software (release 14).
Results
Dietary fiber.
The dietary fiber concentrations in WGB and malt were 15.4 and 12.1 g/100 g dry weight, respectively, whereas that in BSG was 3–4 times higher (58.2 g/100 g dry weight) (Table 1). Most of the dietary fiber was insoluble and the proportion of insoluble fiber polysaccharides was higher in BSG (98%) than in malt (89%) and WGB (83%; P < 0.05) (Table 2). The main components of the dietary fiber polysaccharides were glucose (35–48%), xylose (29–35%), and arabinose (16–22%). Dietary fiber polysaccharides in WGB were more fermented by the rats than those in BSG, which were more fermented than those in malt (P < 0.001). All the main components were less fermented in rats given malt than in those given WGB (P < 0.001). Of the glucose-containing dietary fiber polysaccharides, 70% was excreted in feces by rats fed malt, 57% by those fed BSG, and only 36% by those given WGB, where all 3 groups differed from one another (P < 0.05). L. rhamnosus had no effect on the degree of fermentation.
TABLE 2.
Composition and fecal excretion of dietary fiber components in rats fed WGB, malt, or BSG diet with or without Lactobacillus rhamnosus 271 for 12 d1
| WGB -Lr/Lr |
Malt -Lr/Lr |
BSG -Lr/Lr |
2-Way ANOVA P-values |
||||||
| Dietary fiber composition | Fecal excretion | Dietary fiber composition | Fecal excretion | Dietary fiber composition | Fecal excretion | Fiber | Lr | Fiber×Lr | |
| g/100 g dry wt (% soluble fiber) | % of intake | g/100 g dry wt (% soluble fiber) | % of intake | g/100 g dry wt (% soluble fiber) | % of intake | ||||
| Rhamnose | tr2 | tr | tr | tr | tr | tr | _ | _ | _ |
| Arabinose | 2.0 (6) | 34 ± 1a | 1.7 (13) | 38 ± 1b | 7.7 (2) | 36 ± 1ab | 0.005 | NS3 | NS |
| Xylose | 3.5 (3) | 42 ± 1a | 2.7 (9) | 49 ± 1b | 12.1 (1) | 45 ± 1ab | 0.005 | NS | NS |
| Mannose | 0.3 (14) | 19 ± 1a | 0.2 (21) | 34 ± 1b | 0.5 (4) | 39 ± 1b | <0.001 | NS | NS |
| Galactose | 0.2 (16) | 69 ± 2a | 0.2 (29) | 67 ± 1a | 1.1 (6) | 53 ± 1b | <0.001 | NS | NS |
| Glucose | 5.9 (29) | 35 ± 1a | 2.7 (8) | 70 ± 2b | 13.8 (2) | 58 ± 1c | <0.001 | NS | NS |
| Uronic acid | 0.3 (17) | 60 ± 2 | 0.3 (12) | 56 ± 1 | 1.3 (7) | 60 ± 1 | NS | NS | NS |
| Dietary fiber polysaccharides | 12.2 (17) | 38 ± 1a | 7.8 (11) | 55 ± 1b | 36.5 (2) | 49 ± 1c | <0.001 | NS | NS |
| Lignin | 3.2 | nd4 | 4.3 | nd | 21.5 | nd | _ | _ | _ |
Values are means ± SEM, = 6–7 or mean percentages, n = 2. For percent intake, means in a row without a common letter differ, P < 0.05.
tr, trace amounts, < 0.05 g/100 g.
NS, Not significant, ≥ 0.05.
nd, Not determined.
Feed intake and weight gain.
The rats in the different dietary groups ate most of the diet provided (95–99%). The increase in body weight during the 5-d experimental period and the cecal pH at the end of the experiment did not differ between the rats given the different barley products, whereas cecal content, the weight of the cecal tissue, and the bulking capacity differed (Table 3). Thus, the cecal content was higher in rats fed malt than in those fed BSG (P < 0.002) and the bulking capacity was higher with malt than with BSG and WGB (P < 0.001). The cecal tissue weight was higher in rats fed WGB than in those given BSG (P < 0.011). When probiotics were added to the diets, the increase in body weight was greater (P = 0.006), whereas the cecal pH was lower (P = 0.001).
TABLE 3.
Increase in body weight, weight of cecal content and tissue, cecal pH, and bulking capacity in rats fed WGB, malt, and BSG diet with or without Lactobacillus rhamnosus 271 for 12 d1
| WGB |
Malt |
BSG |
2-Way ANOVA P-values |
||||||
| -Lr | +Lr | -Lr | +Lr | -Lr | + Lr | Fiber | Lr | Fiber×Lr | |
| Feed efficiency,2g gain/g feed | 0.21 ± 0.01 | 0.25 ± 0.03 | 0.17 ± 0.01 | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.25 ± 0.02 | NS3 | 0.006 | NS |
| Cecal content, g | 1.8 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | 0.0024 | NS | NS |
| Cecal tissue weight, g | 0.56 ± 0.01 | 0.63 ± 0.01 | 0.57 ± 0.02 | 0.58 ± 0.03 | 0.53 ± 0.02 | 0.54 ± 0.02 | 0.0115 | NS | NS |
| Cecal pH | 7.1 ± 0.1 | 6.7 ± 0.1 | 7.0 ± 0.0 | 6.8 ± 0.1 | 7.1 ± 0.1 | 7.0 ± 0.1 | NS | 0.001 | NS |
| Bulking capacity, g/g fiber ingested | 0.8 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | <0.0016 | NS | NS |
Values are means ± SEM, = 6–7.
Measured over 5 d.
NS, Not significant, ≥0.05.
Values for groups fed malt were significantly higher than those fed BSG.
Values for groups fed WGB were significantly higher than those fed BSG.
Values for groups fed malt were significantly higher than those fed WGB and BSG.
The formation of CA in the hindgut of rats.
Total cecal concentrations of CA did not differ between rats fed the 3 barley products, whereas the cecal pool was greater in rats fed malt than in rats fed BSG (P = 0.009) (Table 4). Of the individual acids, the cecal concentration of propionic acid was greater in rats fed malt than in those fed BSG (P = 0.021), whereas that of lactic acid was lower in rats fed WGB than in those fed BSG (P = 0.013). When L. rhamnosus was included in the diet, the cecal concentration of individual CA was greater than without the bacterium, except for lactic and succinic acids. As a consequence, the total CA concentrations and pools were also greater with L. rhamnosus than without (P ≤ 0.010).
TABLE 4.
Cecal pools and concentrations of CA in the cecum and distal colon in rats fed WGB, malt and BSG diet with or without Lactobacillus rhamnosus 271 for 12 d1
| WGB |
Malt |
BSG |
2-Way ANOVA P-values |
||||||
| -Lr | +Lr | -Lr | +Lr | -Lr | +Lr | Fiber | Lr | Fiber×Lr | |
| Cecum, μmol/g | |||||||||
| Acetic | 39 ± 1 | 55 ± 7 | 49 ± 3 | 61 ± 6 | 41 ± 4 | 51 ± 3 | NS3 | 0.001 | NS |
| Propionic | 9 ± 1 | 13 ± 1 | 11 ± 0 | 13 ± 1 | 9 ± 1 | 11 ± 1 | 0.0214 | <0.001 | NS |
| Butyric | 7 ± 0 | 18 ± 5 | 12 ± 1 | 18 ± 3 | 9 ± 1 | 12 ± 2 | NS | 0.001 | NS |
| Lactic | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.0135 | NS | NS |
| Succinic | 2 ± 0 | 2 ± 0 | 2 ± 0 | 1 ± 0 | 2 ± 0 | 1 ± 0 | NS | NS | NS |
| Minor2 | 4 ± 0a | 6 ± 1b | 5 ± 0ab | 5 ± 0ab | 5 ± 0ab | 5 ± 0ab | NS | 0.010 | 0.026 |
| Total | 61 ± 2 | 94 ± 13 | 79 ± 4 | 98 ± 10 | 66 ± 6 | 80 ± 5 | NS | 0.001 | NS |
| Total pool, μmol | 116 ± 11 | 228 ± 41 | 173 ± 10 | 225 ± 34 | 123 ± 20 | 141 ± 6 | 0.0094 | 0.002 | NS |
| Distal colon, μmol/g | |||||||||
| Acetic | 37 ± 1a | 39 ± 3a | 38 ± 2a | 46 ± 2b | 37 ± 5a | 29 ± 2a | 0.0046 | NS | 0.013 |
| Propionic | 8 ± 0a | 10 ± 1b | 10 ± 1b | 13 ± 1c | 8 ± 1a | 7 ± 0a | <0.0014 | NS | 0.015 |
| Butyric | 6 ± 0 | 8 ± 1 | 9 ± 1 | 10 ± 1 | 6 ± 1 | 6 ± 0 | <0.0016 | NS | NS |
| Minor2 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 3 ± 0 | 0.0447 | NS | NS |
| Total | 55 ± 1a | 61 ± 4ab | 61 ± 3b | 73 ± 4c | 55 ± 6a | 45 ± 3a | <0.0016 | NS | 0.016 |
Values are means ± SEM, = 5–7. Means in a row without a common letter differ, P < 0.05.
Sum of iso-butyric, iso-valeric, valeric, caproic, and heptanoic acids.
NS, Not significant, ≥ 0.05.
Values for groups fed malt were significantly higher than those fed BSG.
Values for groups fed BSG were significantly higher than those fed WGB.
Values for groups fed malt were significantly higher than those fed WGB and BSG.
Values for groups fed malt and WGB were significantly higher than those fed BSG.
The concentrations of CA in the proximal colon did not differ among the groups (data not shown). In the distal part of the colon, the type of barley product had a considerable effect on the total and individual CA concentrations, with malt generally leading to higher concentrations than the other 2 products (P ≤ 0.044). The concentration of propionic acid was higher in rats fed malt than in those fed BSG, and the butyric acid concentration was higher than in those fed BSG and WGB (P < 0.001). There were also some interactions between the barley product and the probiotic, and concentrations of acetic and propionic acid were greater in rats fed malt with L. rhamnosus than with malt only (P < 0.05). The concentration of propionic acid was also higher in rats given WGB and L. rhamnosus than in rats fed WGB only (P < 0.05). There were no effects of L. rhamnosus in combination with BSG.
Concentrations of SCFA in portal serum.
Acetic acid (980–1600 μmol/L), propionic acid (72–200 μmol/L), and butyric acid (46–210 μmol/L) were the major acids in the portal serum (Table 5). Rats fed malt had higher concentrations of propionic and butyric acids in portal serum than rats given WGB and BSG (P ≤ 0.03). The concentration of isobutyric acid also differed with the type of barley given (P < 0.003), and BSG gave a lower concentration than malt. When probiotics were added to the diets, the concentrations of individual acids in portal serum were greater, apart from acetic acid.
TABLE 5.
Concentrations of SCFA in portal serum in rats fed WGB, malt and BSG diet with or without Lactobacillus rhamnosus 271 for 12 d1
| WGB |
Malt |
BSG |
2-Way ANOVA P-values |
||||||
| SCFA | -Lr | +Lr | -Lr | +Lr | -Lr | +Lr | Fiber | Lr | Fiber×Lr |
| μmol/L | |||||||||
| Acetic | 1200 ± 95 | 1200 ± 160 | 1200 ± 94 | 1600 ± 310 | 980 ± 35 | 1200 ± 210 | NS3 | NS | NS |
| Propionic | 77 ± 10 | 130 ± 24 | 130 ± 18 | 200 ± 56 | 72 ± 7 | 100 ± 23 | 0.0284 | 0.043 | NS |
| Butyric | 46 ± 5 | 110 ± 27 | 96 ± 14 | 210 ± 84 | 53 ± 7 | 83 ± 22 | 0.0234 | 0.009 | NS |
| Isobutyric | 15 ± 1 | 22 ± 3 | 21 ± 3 | 32 ± 5 | 13 ± 1 | 17 ± 2 | 0.0035 | 0.006 | NS |
| Isovaleric | 16 ± 1 | 25 ± 3 | 21 ± 2 | 25 ± 3 | 16 ± 3 | 19 ± 2 | NS | 0.016 | NS |
| Valeric | 6 ± 1 | 13 ± 3 | 11 ± 2 | 16 ± 4 | 5 ± 1 | 8 ± 2 | NS | 0.026 | NS |
| Minor2 | 37 ± 2 | 60 ± 9 | 53 ± 6 | 73 ± 7 | 35 ± 5 | 45 ± 6 | 0.0045 | 0.002 | NS |
| Total | 1400 ± 100 | 1600 ± 190 | 1500 ± 130 | 2200 ± 450 | 1200 ± 45 | 1500 ± 260 | NS | NS | NS |
Values are means ± SEM, = 5–7.
Sum of caproic and heptanoic acids.
NS, Not significant, ≥ 0.05.
Values for groups fed malt were significantly higher than those fed WGB and BSG.
Values for groups fed malt were significantly higher than those fed BSG.
Composition of amino acids and NH4 in barley products.
The total amounts of amino acids in WGB and malt were 9.7 and 9.5 g/100 g dry weight, respectively, whereas BSG contained ~1.5 times more amino acids (24.3 g/100 g dry weight) (Supplemental Table 2). The amount of protein analyzed by the combustion method was in the same range (Table 1). All the barley products exhibited the same amino acid patterns, showing the greatest proportion of glutamic acid (20–23%), followed by proline (10–12%), leucine (7–8%), and asparagine (6–7%).The contribution of amino acids from malt and WGB was higher in the diets than that from BSG.
Concentrations and proportions of amino acids and NH4 in portal plasma.
Neither the total concentration nor the individual concentration of amino acids and NH4 in portal plasma differed among the groups, although the proportions were different. Detectable amounts of 19 amino acids and NH4 were found in all the dietary groups. Results concerning significant differences in proportions (serine, glutamic acid, glutamine, tyrosine, histidine, and NH4) are given in Supplemental Table 3. Both the type of barley product and the addition of the probiotic strain affected the proportions of these amino acids. Plasma from rats given WGB and BSG contained greater proportions of glutamine than the groups given malt (P = 0.03). Further, rats given BSG had greater proportions of serine in their plasma than rats fed the other 2 barley products (P = 0.008). The proportion of NH4 was lower in plasma from rats fed WGB and BSG than in those fed malt (P = 0.001). The addition of the probiotic strain increased the proportions of glutamic acid, tyrosine, and NH4 in plasma (P ≤ 0.04).
Bacteriology.
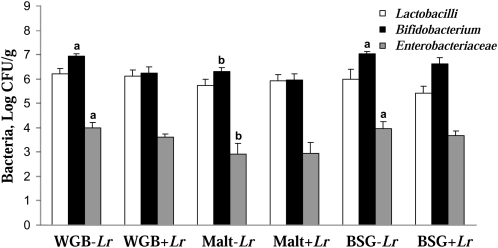
The viable cecal counts of bifidobacteria and Enterobacteriaceae in rats fed malt were lower than in rats given WGB (P = 0.003) or BSG (P = 0.006) (Fig. 1). Diet groups containing L. rhamnosus 271 had less bifidobacteria (P = 0.004).
FIGURE 1.
Bacterial counts of Lactobacillus, Bifidobacterium, and Enterobacteriaceae in the cecum contents in rats fed WGB, malt, and BSG diet with or without Lactobacillus rhamnosus 271 for 12 d. Values are means ± SEM, n = 6–7. Means without a common letter differ, P < 0.05.
Discussion
Feeding rats diets containing malt led to higher concentrations of butyric acid in the distal part of colon and the portal serum than feeding diets containing WGB and BSG. Malt gave similar results for propionic acid in serum. The higher distal concentrations of butyric acid are of particular interest, because this acid may have potential benefits for the colonic mucosa and colonic diseases, such as ulcerative colitis and colon cancer, occurring in this part of the colon. Moreover, increased concentrations of both butyric and propionic acids in the circulation are associated with metabolic effects (27, 28). It has been suggested that these acids may modulate proinflammatory markers, thus affecting the mechanisms associated with metabolic disorders (27, 28).
The bifidobacteria count was lower in rats fed the malt diet than the other barley diets. Although the bacteria counted in this study do not account for the whole microbiota, they provide indications of changes in the populations. A change in the microbiota may also result in other pathways for fermentation and a change in the degradation products formed, as judged by the higher concentrations of butyric acid in the distal colon of rats fed malt than in those fed the other barley products. We speculate that the change in cecal microbiota in rats fed malt also facilitated the transportation of propionic and butyric acids through the mucosa. The importance of the composition of the microbiota in this context is further supported by the fact that the concentrations of these acids were higher in both the cecum and portal serum when L. rhamnosus was added to the diets. A higher concentration of propionic and butyric acids in serum has previously been reported when probiotics were added to a blueberry husk diet (26). However, the concentrations of butyric and propionic acids in the cecum and serum of rats were considerably lower than when using the barley products in this study (26). A change in the composition of the microbiota following the addition of probiotics was also reflected by the pH, which was lower in the groups given L. rhamnosus. It is worth mentioning that the growth of potential pathogens, such as Clostridia, is reduced at low pH, and L. rhamnosus 271 may thus be able to counteract the growth of pathogenic species (29).
The cecal pools of CA were higher in rats fed malt than in those fed WGB or BSG, although the fiber polysaccharides in malt seemed to be fermented less than those in the other barley products studied. A possible explanation for this may be that the fiber in WGB is degraded to a lower degree of polymerization (< ~20) during the malting process and is consequently not precipitated in ethanol during the dietary fiber analysis, giving too low an apparent fiber content. If this is the case, the dietary fiber intake of rats fed malt was in fact higher than with the other barley products, also explaining the formation of more CA. If the fiber content in malt is assumed to be the same as that in WGB, the degree of fermentation of these 2 barley products also is very similar (36 vs. 38%). BSG was the least fermented (P < 0.05) of the 3 barley products, which was probably due to the high content of lignin and lower amounts of fermentable polymers containing glucose (β-glucans). The cellulose-containing polysaccharides were probably enriched in the brewing process and these are known to be poorly fermented.
There are some difficulties in analyzing glutamine in test materials. During acid hydrolysis, glutamine is converted into glutamic acid and the amount of glutamine measured therefore also includes the glutamic acid content of the sample. However, according to Kanauchi and Agata (8), only traces of glutamic acid are present in GBF and most of the glutamic acid measured thus arises from glutamine. Further, the glutamine in barley has been reported to be bound to the dietary fiber and thus reaches the colon where it is liberated after fiber fermentation, becoming a substrate for the colonic mucosa. However, glutamine is a very fragile amino acid, and most of it not bound to dietary fiber is degraded by gastric acid (8).
The total protein content was nearly 1.5 times higher in BSG than in malt or WGB, while the composition of the protein was very similar in all 3 products. However, the contribution of protein and glutamine (see above) from the barley products in the diets was highest with malt (Supplemental Table 2). Despite this, the total concentrations of amino acids in the portal plasma did not differ, and rats fed malt had an even lower proportion of glutamine in plasma than the groups fed WGB and BSG. An increased formation of butyric acid in the colon may reduce the need for glutamine, thereby increasing the amount of glutamine in the circulation (4). Although malt led to high amounts of propionic and butyric acid in the hindgut and serum of rats, the concentration of glutamine in plasma was not higher, indicating that the comparatively large amounts of glutamine present in malt do not reach the colon. This was further supported by the fact that the addition of blueberry husks to a diet containing casein as protein source was shown to give similar plasma concentrations of glutamine to those in this study, using the same animal model (C. Bränning, Å. Håkansson, S. Ahrné, B. Jeppsson, G. Molin, M. Nyman, unpublished data). The beneficial effects of GFB in patients with ulcerative colitis and in rats with DSS-induced colitis have been partly ascribed to glutamine (9). The lower plasma concentrations of glutamine in rats fed malt may be connected to the malting process in some way. As discussed above, it appears that the dietary fiber was degraded during malting, which may result in lower amounts of bound glutamine.
The proportion of NH4 in plasma in the rats fed malt was higher than in the WGB and BSG groups. The origin of ammonia in the portal vein is complex, but some arises from the metabolism of glutamine (30). This may be another reason for the lower proportion of glutamine in the rats given malt. Previous studies in humans have shown that an increase in the number of fecal bifidobacteria was associated with a reduction in the concentration of NH4 in feces and blood (31). Bifidobacterium spp. use NH4 as a nitrogen source for their growth (30). Rats fed malt had a lower viable count of cecal bifidobacteria than those given the other 2 barley products, which could explain the higher concentration of NH4 found in the portal plasma.
In conclusion, a diet containing malt led to higher concentrations of butyric acid in the distal colon and in portal serum of rats than in those of rats fed diets containing WBG and BSG. The effects on propionic acid were comparable to those on butyric acid. The concentration of glutamine in plasma was similar for all barley products, whereas the proportion of glutamine was lower in rats fed malt. Furthermore, the number of bifidobacteria was lower. The formation of butyric acid increased when the probiotic strain L. rhamnosus 271 was added to the barley products, whereas the number of bifidobacteria decreased. No effect on glutamine was seen with this strain. Thus, it appears that the combination of malt and L. rhamnosus has the potential to have particularly beneficial effects on the colon mucosa, and possibly also the metabolism, due to the high level of butyric acid formation. It remains to be determined whether these effects also are valid in humans.
Supplementary Material
Acknowledgments
C.B. conducted the research; and C.B. and M.N. designed the studies, analyzed the data, wrote the paper, and were responsible for final content. Both authors read and approved the final manuscript.
Footnotes
Supported by the Functional Food Science Centre at Lund University, Sweden.
Supplemental Tables 1–3 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BSG, Brewer’s spent grain; CA, carboxylic acid; DSS, dextran sulfate sodium; GBF, germinated barley foodstuff; WGB, whole grain barley.
Literature Cited
- 1.Fleming LL, Floch MH. Digestion and absorption of fiber carbohydrate in the colon. Am J Gastroenterol. 1986;81:507–11 [PubMed] [Google Scholar]
- 2.Karinch AM, Pan M, Lin CM, Strange R, Souba WW. Glutamine metabolism in sepsis and infection. J Nutr. 2001;131:S2535–8 [DOI] [PubMed] [Google Scholar]
- 3.Wu GY, Field CJ, Marliss EB. Glutamine and glucose metabolism in rat splenocytes and mesenteric lymph node lymphocytes. Am J Physiol. 1991;260:E141–7 [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DJ, Kendall CW, Vuksan V. Inulin, oligofructose and intestinal function. J Nutr. 1999;129:S1431–3 [DOI] [PubMed] [Google Scholar]
- 5.Berggren AM, Björck IME, Nyman EMG, Eggum BO. Short-chain fatty acid content and pH in caecum of rats given various sources of carbohydrates. J Sci Food Agric. 1993;63:397–406 [Google Scholar]
- 6.Kanauchi O, Nakamura T, Agata K, Mitsuyama K, Iwanaga T. Effects of germinated barley foodstuff on dextran sulfate sodium-induced colitis in rats. J Gastroenterol. 1998;33:179–88 [DOI] [PubMed] [Google Scholar]
- 7.Lambo-Fodje AM, Oste R, Nyman ME. Short-chain fatty acid formation in the hindgut of rats fed native and fermented oat fibre concentrates. Br J Nutr. 2006;96:47–55 [DOI] [PubMed] [Google Scholar]
- 8.Kanauchi O, Agata K. Protein, and dietary fiber-rich new foodstuff from brewer's spent grain increased excretion of feces and jejunum mucosal protein content in rats. Biosci Biotechnol Biochem. 1997;61:29–33 [DOI] [PubMed] [Google Scholar]
- 9.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–4 [PubMed] [Google Scholar]
- 10.Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Tomiyasu N, Nishiyama T, Tateishi H, Shirachi A, Ide M, et al. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12:1225–30 [DOI] [PubMed] [Google Scholar]
- 11.Goldammer T. The brewers' handbook. The complete book to brewing beer. 2nd ed Clifton (VA): Apex Publishers; 2000 [Google Scholar]
- 12.Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85:88–94 [DOI] [PubMed] [Google Scholar]
- 13.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–74 [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92:2400–8 [DOI] [PubMed] [Google Scholar]
- 15.Björck I, Nyman M, Pedersen B, Siljeström M, Asp N-G, Eggum BO. Formation of enzyme resistant starch during autoclaving of wheat starch: studies in vitro and in vivo. J Cereal Sci. 1987;6:159–72 [Google Scholar]
- 16.Eggum BO. A study of certain factors influencing protein utilization in rats and pigs [PhD thesis]. Copenhagen: National Institute of Animal Science; 1973 [Google Scholar]
- 17.Wiles PG, Gray IK, Kissling RC. Routine analysis of proteins by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. J AOAC Int. 1998;81:620–32 [PubMed] [Google Scholar]
- 18.Björck I, Siljeström M. In vivo and in vitro digestibility of starch in autoclaved pea and potato products. J Sci Food Agric. 1992;58:541–53 [Google Scholar]
- 19.Asp N-G, Johansson C-G, Hallmer H, Siljeström M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31:476–82 [DOI] [PubMed] [Google Scholar]
- 20.Theander O, Åman P, Westerlund E, Andersson R, Pettersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J AOAC Int. 1995;78:1030–44 [PubMed] [Google Scholar]
- 21.Zhao G, Nyman M, Jönsson JÅ. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr. 2006;20:674–82 [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, Liu JF, Nyman M, Jönsson JÅ. Determination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatography. J Chromatogr B. 2007;846:202–8 [DOI] [PubMed] [Google Scholar]
- 23.Åssveen M. Amino acid composition of spring barley cultivars used in Norway. Acta Agriculturae Scandinavica, Section B - Plant Soil Science. 2009;59:395–401 [Google Scholar]
- 24.Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53 [DOI] [PubMed] [Google Scholar]
- 25.Stenberg M, Marko-Varga G, Öste R. Enantioseparation of d- and l-amino acids by a coupled system consisting of an ion-exchange column and a chiral column and determination of d-aspartic acid and d-glutamic acid in soy products. Food Chem. 2002;79:507–12 [Google Scholar]
- 26.Bränning C, Håkansson Å, Ahrné S, Jeppsson B, Molin G, Nyman M. Blueberry husks and multi-strain probiotics affect colonic fermentation and weight gain in healthy rats. Br J Nutr. 2009;101:859–70 [DOI] [PubMed] [Google Scholar]
- 27.Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19:71–84 [DOI] [PubMed] [Google Scholar]
- 28.Nilsson A, Ostman E, Preston T, Bjorck I. Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr. 2008;62:712–20 [DOI] [PubMed] [Google Scholar]
- 29.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64 [DOI] [PubMed] [Google Scholar]
- 30.Deguchi Y, Makino K, Iwabuchi A, Watanuki M, Yamashita T. Selection of ammonia-assimilating bifidobacteria and their effect on ammonia levels in rat caecal contents and blood. Microb Ecol Health Dis. 1993;6:85–94 [Google Scholar]
- 31.Zhao HY, Wang HJ, Lu Z, Xu SZ. Intestinal microflora in patients with liver cirrhosis. Chin J Dig Dis. 2004;5:64–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.