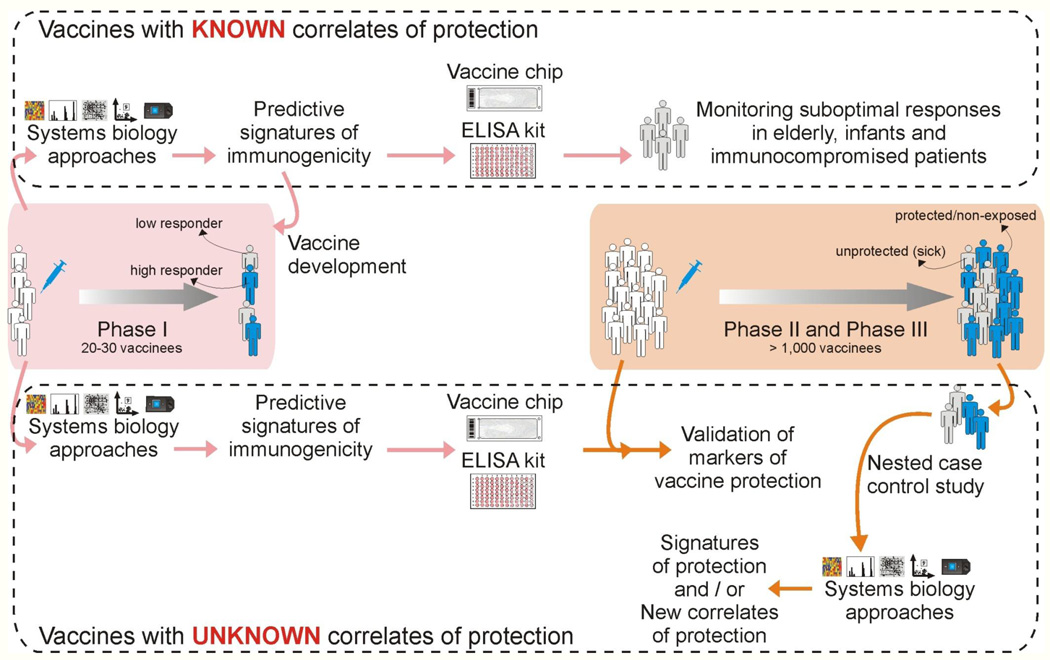
Figure 3. Integrating systems biology approaches into clinical trials.
Top: For vaccines for which correlates or protection are known (Table 1), systems approaches can be used to identify early signatures of protection in a phase 1 trial. The key genes from these signatures can be incorporated into a vaccine chip or ELISA kit, which can then be used to identify non responders or sub-optimal responders, particularly in special populations such as immunocompromised patients, elderly and infants. Bottom: For new and emerging vaccines, for which correlates of protection were unknown, signatures that predict various aspects of immunogenicity (e.g. CD8+ T cell responses or neutralizing antibody responses) can be assessed in phase I trials. Such signatures can then be incorporated into a vaccine chip or ELISA kit that can then be used in phase II and III trials to determine their capacity to predict protection. Alternatively, a retrospective nested case controlled study could be done in a phase II and III trial to identify signatures of protection.