Abstract
Tropical forest canopies house most of the globe's diversity, yet little is known about global patterns and drivers of canopy diversity. Here, we present models of ant species density, using climate, abundance and habitat (i.e. canopy versus litter) as predictors. Ant species density is positively associated with temperature and precipitation, and negatively (or non-significantly) associated with two metrics of seasonality, precipitation seasonality and temperature range. Ant species density was significantly higher in canopy samples, but this difference disappeared once abundance was considered. Thus, apparent differences in species density between canopy and litter samples are probably owing to differences in abundance–diversity relationships, and not differences in climate–diversity relationships. Thus, it appears that canopy and litter ant assemblages share a common abundance–diversity relationship influenced by similar but not identical climatic drivers.
Keywords: Formicidae, species richness, global diversity gradients
1. Introduction
Tropical forest canopies may house more than half of the world's animal species (Erwin 1982; Stork 1993; Ødegaard 2000; Novotny et al. 2002), but little is known about how canopy diversity varies at global scales (see Kitching et al. 1993; Majer et al. 2001). If the patterns and the climatic correlates of canopy diversity are different from ground-dwelling taxa, current models (which are largely based on ground-dwelling taxa) may not apply for a striking majority of the Earth's biodiversity. Alternatively, if similar factors drive canopy and ground-dwelling species diversity, then understanding the factors that shape the diversity of ground-dwelling taxa will be useful for understanding canopy diversity as well.
Ants can comprise more than half of the arthropod abundance and biomass of tropical forest canopies (e.g. Fittkau & Klinge 1973; Tobin 1995; Floren & Linsenmair 1997; Davidson et al. 2003). It is clear that climate is correlated with ant diversity, with the combination of temperature and precipitation often representing the best two climatic predictors for the diversity of litter-dwelling ants (Kaspari et al. 2004; Sanders et al. 2007; Dunn et al. 2009). However, different factors may limit the diversity of litter and canopy ants. Canopy ants tend to feed at lower trophic levels than litter ants (Yanoviak & Kaspari 2000; Blüthgen et al. 2003; Davidson et al. 2007) and therefore may depend more directly on plant production. As plant productivity is highest in warm, wet and aseasonal environments (e.g. Schuur 2003), canopy ant diversity may be more strongly associated with precipitation and temperature than litter ant diversity. Additionally, if canopy ants maintain large colony sizes relative to litter ants (Davidson et al. 2007), then a given number of workers may be distributed among fewer canopy species, leading to different abundance–diversity relationships. Finally, canopy ants potentially face greater exposure to climatic variability (e.g. Hood & Tschinkel 1990) than litter ants, which may lead to greater dependence of canopy diversity on climatic seasonality.
Here, we generate models of ant species density (i.e. S = the number of species in a sample; Gotelli & Colwell 2001) that use climate, abundance and stratum (i.e. 23 canopy versus 192 litter collections) to understand how and how well these variables predict ant species density.
2. Data
We compiled data on canopy ant species density from the literature using studies that sampled arboreal assemblages by canopy fogging. Fogging studies attempt to sample only ants present in the canopy, but this does not necessarily exclude ground-nesting, canopy-foraging species. We recorded species density (S) and abundance (N denotes the number of individual ants), and for studies that did not differentiate spatially between locations, we used the mean of each variable. Twenty-three localities met the above criteria (see electronic supplementary material, table S1). To compare canopy patterns with better understood patterns of litter ants (e.g. Kaspari et al. 2000, 2004; Sanders et al. 2007; Dunn et al. 2009), we extracted similar data from 192 litter samples (i.e. the subset of Winkler extractions from forested areas from Dunn et al. (2009) that reported abundance; see electronic supplementary material, table S2). For each location, we extracted mean annual temperature, annual precipitation (hereafter, ‘temperature’ and ‘precipitation’), annual temperature range and precipitation seasonality (i.e. the coefficient of variation of monthly precipitation) from WorldClim (Hijmans et al. 2005). All climatic predictor variables were converted to z-scores.
3. Analyses and results
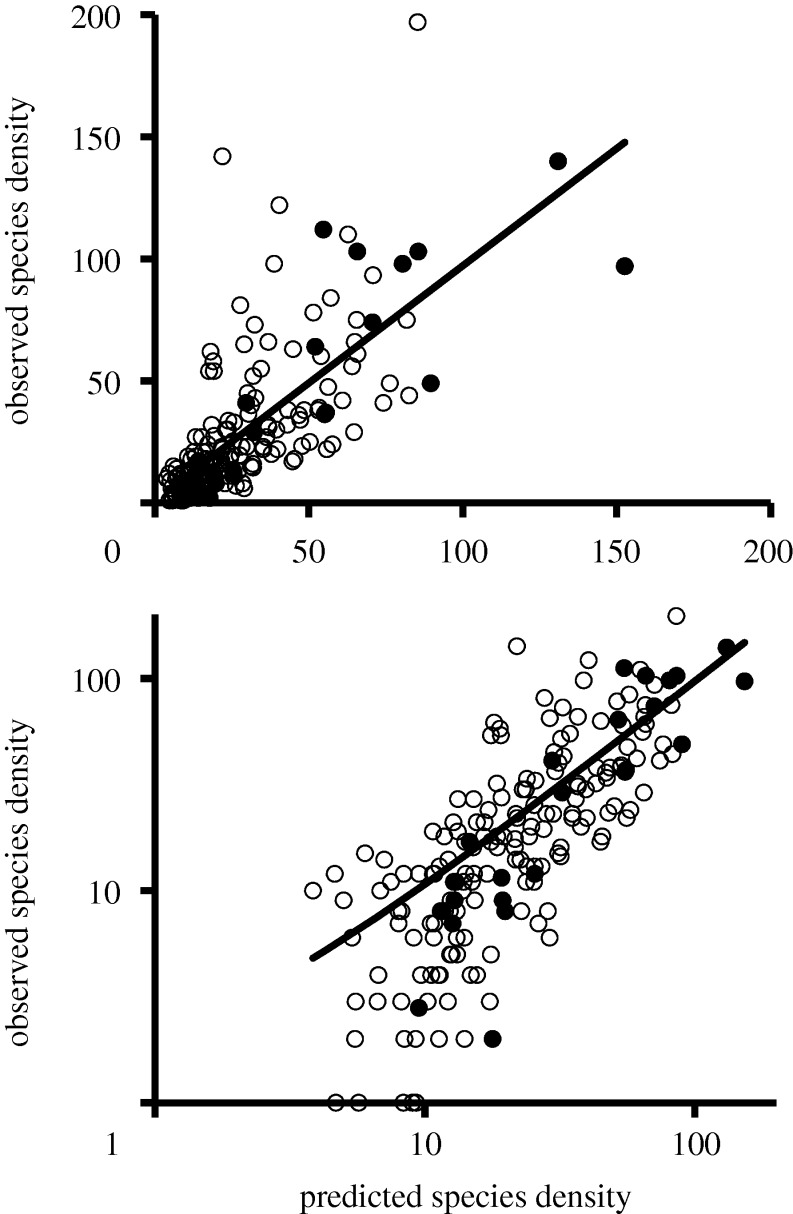
We combined canopy and litter samples and made three nested generalized linear models to predict S, using (i) climatic variables; (ii) climate plus canopy/litter; and (iii) climate, canopy/litter and abundance. Climate contributed significantly (all but one climatic effect test was significant; table 1) and similarly (i.e. the confidence intervals of the parameter estimates overlapped; table 1) to all three models. Temperature, precipitation and abundance were all positively correlated with species density in all three models, while temperature range and precipitation seasonality were either negatively correlated with or were not significant predictors of ant species density (table 1). Both of the more complex models performed better, based on AIC scores than the simpler models (table 1). Model predictions of species density accounted for 52 per cent of the variation in the combined observed data and 73 per cent of the variation in canopy species density (figure 1).
Table 1.
Three nested generalized linear models of species density with all samples combined. The first model (‘climate’) includes the climatic parameters. ‘+ canopy/litter’ adds the classification variable (whether the samples are from the canopy or litter). ‘+abundance’ adds abundance (N) as a measure of sampling effort. All climatic variables have been converted to z-scores. The two more complex models are significant given AIC minimization. Note that the effect of canopy/litter is significant, unless the number of individuals is included. Parameter estimates and AIC values do not include non-significant terms.
| climate |
canopy/litter |
abundance |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| estimates | 84% CI |
estimates | 84% CI |
estimates | 84% CI |
||||
| intercept | 3.075** | 3.050 | 3.100 | 3.056** | 3.029 | 3.082 | 3.063** | 3.039 | 3.088 |
| mean annual temperature | 0.514** | 0.468 | 0.561 | 0.513** | 0.466 | 0.556 | 0.565** | 0.535 | 0.595 |
| annual precipitation | 0.208** | 0.182 | 0.234 | 0.217** | 0.192 | 0.243 | 0.246** | 0.224 | 0.268 |
| temperature range | −0.130** | −0.181 | −0.079 | −0.105** | −0.157 | −0.054 | n.s. | n.s. | n.s. |
| precipitation seasonality | −0.115** | −0.141 | −0.089 | −0.096** | −0.123 | −0.070 | −0.095** | −0.120 | −0.070 |
| canopy/litter (canopy) | — | 0.169* | 0.115 | 0.222 | n.s. | n.s. | n.s. | ||
| number of individuals | — | — | 0.135** | 0.125 | 0.144 | ||||
| AIC | − 28 259 | − 28 278 | − 28 528 | ||||||
| r2 observed ∼ predicted | 0.452 | 0.465 | 0.522 | ||||||
*p < 0.01.
**p < 0.0001.
n.s., not significant at p = 0.05.
Figure 1.
Ant species density predicted by our model (i.e. the ‘+abundance’ model in table 1) compared with the observed ant species density. Open circles represent litter samples, closed circles represent canopy fogging samples. Both panels present the same information, with the bottom panel scaled with log10-transformed axes (to allow visualization). The line represents the ordinary least-squares regression on the combined dataset with observed = 1.1 + (0.96 × predicted), p < 0.0001, r2 = 0.52, n = 192. The relationship for the canopy data is observed = 1.8 + (0.93 × predicted), p < 0.0001, r2 = 0.73, n = 23.
To investigate whether the effects of the predictor variables differed between canopy and litter samples, we created three (non-nested) generalized linear models separately, adding the interaction term for canopy/litter × temperature, canopy/litter × precipitation and canopy/litter × abundance. All three interaction terms were significant (table 2) and the confidence intervals for most parameter estimates overlapped (excepting precipitation in the precipitation–interaction model; table 2) with the ‘+abundance’ model (i.e. the best model without interaction terms). Of the six models presented here, the ‘best’ model (i.e. the lowest AIC score) includes the effects of temperature, precipitation, precipitation seasonality, abundance and the abundance–canopy/litter interaction.
Table 2.
Three (non-nested) generalized linear models of species density with all samples combined. All three models include climatic variables, canopy/litter, abundance and the interaction of canopy/litter and temperature, precipitation and abundance, respectively. The effect sizes for the interaction terms are for canopy samples (with litter samples being that value×−1). Thus, the effects of mean annual temperature, annual precipitation and abundance differ between canopy and litter samples. Parameter estimates and AIC values do not include non-significant terms (i.e. for the abundance model).
| interaction of canopy/litter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| temperature |
precipitation |
abundance |
|||||||
| estimates | 84% CI |
estimates | 84% CI |
estimates | 84% CI |
||||
| intercept | 3.088** | 3.062 | 3.115 | 3.084** | 3.058 | 3.109 | 3.076** | 3.034 | 3.118 |
| mean annual temperature | 0.489** | 0.441 | 0.537 | 0.512** | 0.467 | 0.558 | 0.556** | 0.504 | 0.607 |
| annual precipitation | 0.209** | 0.182 | 0.236 | 0.193** | 0.165 | 0.221 | 0.243** | 0.205 | 0.281 |
| temperature range | −0.084* | −0.136 | −0.031 | −0.088* | −0.138 | −0.037 | n.s. | n.s. | n.s. |
| precipitation seasonality | −0.106** | −0.133 | −0.079 | −0.110** | −0.136 | −0.084 | −0.094** | −0.137 | −0.051 |
| canopy/litter [canopy] | −1.514** | −1.894 | −1.133 | −0.283** | −0.283 | −0.133 | n.s. | n.s. | n.s. |
| number of individuals | 0.140** | 0.126 | 0.149 | 0.143** | 0.132 | 0.154 | 0.361** | 0.316 | 0.407 |
| interaction of canopy/litter and focal variable | 0.006** | 0.006 | 0.008 | 0.148** | 0.104 | 0.191 | −0.251** | −0.299 | −0.203 |
| AIC | − 28 565 | − 28 549 | − 28 657 | ||||||
| r2 observed ∼ predicted | 0.526 | 0.521 | 0.539 | ||||||
*p < 0.01.
**p < 0.0001.
n.s., not significant at p = 0.05.
4. Discussion
As would be expected (Kaspari et al. 2004; Sanders et al. 2007; Dunn et al. 2009), ant species density was highest in warmer, wetter and relatively stable forests. More important to our goals, three details of the models presented here indicate that species density of canopy and litter ants share similar climatic drivers. First, when considering only climate and habitat, canopy/litter was a significant predictor of species density, but adding abundance to the model made canopy/litter non-significant. Thus, the apparent differences in species density between canopy and litter samples are probably owing to differences in abundance–species density relationships, and not differences in the relationships between climate and species density. Second, the climatic parameter estimates were generally consistent across models that incorporated climate, canopy/litter and abundance, as well as across models with the interactions of temperature, precipitation and abundance with canopy/litter. Finally, the overall model (i.e. the ‘+abundance’ model in table 1), which was generated with disproportionately more litter assemblages, shows a better match between predicted and observed species density for the canopy assemblages (r2 = 0.73, n = 23; figure 1) than it does for the overall dataset (r2 = 0.52, n = 192).
The interaction models indicate differences between canopy and litter species density, but these differences appear relatively minor. The addition of terms for the interactions of canopy/litter and temperature, precipitation and abundance all yielded models that were statistically better than the non-interaction models (based on AIC scores; table 2), but the addition of interaction terms did little to increase the match between predicted and observed species density (i.e. compare the r2 observed ∼ predicted in tables 1 and 2). Our results suggest that these modest differences are a function of differences in the number of individuals sampled. Once abundance was included in the model, the effect of canopy/litter was not a significant predictor of species density. Thus, differences between canopy and litter are probably owing to differences in how climate affects the abundance of canopy versus litter ants and/or how collection methods sample a single abundance–diversity relationship shared by the canopy and litter habitats.
While we argue that the differences in climate–species density relationships between canopy and ground ant are minor, the models which include interaction terms indicate that canopy species density may be more sensitive to the positive effects of temperature and precipitation (i.e. the interaction terms for both predicted higher species density for canopy samples). Additionally, the interaction of canopy/litter and abundance indicates that for a given abundance, canopy samples have fewer species than litter samples (underscoring the potential differences in abundance–species density relationships and supporting the suggestions of Davidson et al. 2007).
While the forest canopy is of great interest to biologists, it remains difficult to study and relatively poorly known. Consequently, canopy biodiversity has played a relatively minor role in understanding and conserving biodiversity. The tendency to date has been to emphasize the differences between canopy and forest floor faunas (e.g. Yanoviak & Kaspari 2000), but here we highlight their similarities. Both faunas increase in species density with increasing temperature, precipitation and climatic stability (Kaspari et al. 2004; Sanders et al. 2007; Dunn et al. 2009) and the differences in their diversity for a given set of climatic conditions appear to be primarily owing to differences in abundances (whether in abundances in samples or abundances per some area or volume). A key remaining question is how best to determine the relevant area or volume over which such abundances should best be considered. If, despite their differences in life history and diet, canopy and litter ants have similar species abundance distributions, it would suggest broad generalities among ant assemblages regardless of whether the ants are walking overhead or underfoot.
Acknowledgements
We thank the two anonymous reviewers for their helpful comments. R.R.D., M.D.W. and N.J.S. were supported by a DOE-NICCR, DOE-PER DE-FG02-08ER64510 and a NASA Biodiversity Grant (ROSES-NNX09AK22G). T.P.M., J.T.L., A.V.S. and B.L.F. by the National Science Foundation (T.P.M. by NSF-OISE-0749047; J.T.L. by NSF-DEB-0640015, Project LLAMA; A.V.S. by NSF-0716966 and B.L.F. by NSF-DEB0842395).
References
- Blüthgen N., Gebauer G., Fiedler K.2003Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137, 426–435 (doi:10.1007/s00442-003-1347-8) [DOI] [PubMed] [Google Scholar]
- Davidson D. W., Cook S. C., Snelling R. R., Chua T. H.2003Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972 (doi:10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- Davidson D. W., Lessard J.-P., Bernau B. C. R., Cook S. C.2007The tropical ant mosaic in a primary Bornean rain forest. Biotropica 39, 468–475 (doi:10.1111/j.1744-7429.2007.00304.x) [Google Scholar]
- Dunn R. R., et al. 2009Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 12, 324–333 (doi:10.1111/j.1461-0248.2009.01291.x) [DOI] [PubMed] [Google Scholar]
- Erwin T. L.1982Tropical forests: their richness in Coleoptera and other species. Coleopterist's Bull. 36, 74–75 [Google Scholar]
- Fittkau E. J., Klinge H.1973On biomass and trophic structure of the Central Amazonian rain forest ecosystem. Biotropica 5, 2–14 (doi:10.2307/2989676) [Google Scholar]
- Floren A., Linsenmair K. E.1997Diversity and recolonization dynamics of selected arthropod groups on different tree species in a lowland rainforest in Sabah, Malaysia with special reference to Formicidae. In Canopy arthropods (eds Stork N. E., Adis J., Didham R. K.), pp. 344–381 New York, NY: Chapman and Hall [Google Scholar]
- Gotelli N. J., Colwell R. K.2001Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391 (doi:10.1046/j.1461-0248.2001.00230.x) [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A.2005Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 25, 1965–1978 (doi:10.1002/joc.1276) [Google Scholar]
- Hood W. G., Tschinkel W. R.1990Desiccation resistance in arboreal and terrestrial ants. Physiol. Ecol. 15, 23–35 [Google Scholar]
- Kaspari M., O'Donnell S., Kercher J. R.2000Energy, density, and constraints to species richness: studies of ant assemblages along a productivity gradient. Am. Nat. 155, 280–293 (doi:10.1086/303313) [DOI] [PubMed] [Google Scholar]
- Kaspari M., Ward P. S., Yuan M.2004Energy gradients and the geographic distribution of local ant diversity. Oecologia 140, 407–414 (doi:10.1007/s00442-004-1607-2) [DOI] [PubMed] [Google Scholar]
- Kitching R. L., Bergelson J. M., Lowman M. D., McIntyre S., Carruthers G.1993The biodiversity of arthropods from Australian rainforest canopies: general introduction, methods, sites and ordinal results. Aust. J. Ecol. 18, 181–191 (doi:10.1111/j.1442-9993.1993.tb00442.x) [Google Scholar]
- Majer J. D., Kitching R. L., Heterick B. E., Hurley K., Brennan K. E. C.2001North–south patterns within arboreal ant assemblages from rain forests in Eastern Australia. Biotropica 33, 643–661 [Google Scholar]
- Novotny V., Basset Y., Miller S. E., Weiblen G. D., Bremerk B., Cizek L., Drozd P.2002Low host specificity of herbivorous insects in a tropical forest. Nature 416, 841–844 (doi:10.1038/416841a) [DOI] [PubMed] [Google Scholar]
- Ødegaard F.2000How many species of arthropods? Erwin's estimate revised. Biol. J. Linn. Soc. 71, 583–597 (doi:10.1111/j.1095-8312.2000.tb01279.x) [Google Scholar]
- Sanders N. J., Lessard J.-P., Dunn R. R., Fitzpatrick M. C.2007Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 16, 640–649 (doi:10.1111/j.1466-8238.2007.00316.x) [Google Scholar]
- Schuur E. A. G.2003Productivity and global climate revisited: the sensitivity of tropical forest growth to precipitation. Ecology 84, 1165–1170 (doi:10.1890/0012-9658(2003)084[1165:PAGCRT]2.0.CO;2) [Google Scholar]
- Stork N. E.1993How many species are there? Biodiv. Conserv. 2, 215–232 (doi:10.1007/BF00056669) [Google Scholar]
- Tobin J. E.1995Ecology and diversity of tropical forest canopy ants. In Forest canopies (eds Lowman M. D., Nadkarni N. M.), pp. 129–147 New York, NY: Academic Press [Google Scholar]
- Yanoviak S. P., Kaspari M.2000Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89, 259–266 (doi:10.1034/j.1600-0706.2000.890206.x) [Google Scholar]