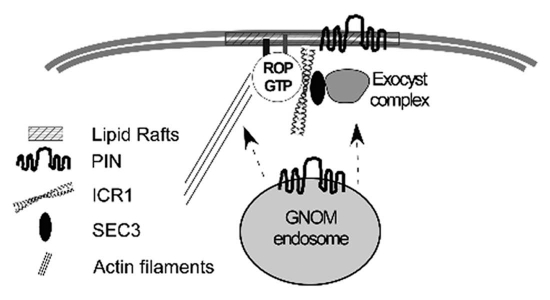
Figure 1.
A model describing the possible involvement of ICR1 in recruitment of PIN proteins to polar domains in the plasma membrane. ICR1 is composed of coiled coil domains and functions as a scaffold that interacts with activated GTP-bound ROPs as well as other proteins.18 Upon GTP bindings, ROPs undergo transient S-acylation and consequent partitioning into lipid rafts.24 Lipid rafts are sterol-rich membrane domains. The involvement of sterols in PIN polarization has been shown.25 ICR1 can interact with the exocyst vesicle tethering subunit SEC3A and ROPs can recruit ICR1-SEC3A complexes to the plasma membrane. In addition, ectopic expression of ICR1 results in cellular deformation similar to that induced by constitutively active ROP mutants. Constitutively active ROPs affect the organization of actin filament and microtubules.16,17,26 Thus, the ICR1 overexpression-induced cell deformation is likely associated with reorganization of the cytoskeleton.18 Collectively, ICR1 may regulate PINs recruitment to polar domains in the plasma membrane19 through its interaction with the exocyst complex. In addition, ICR 1 may affect ROP localization in the membrane and consequently actin organization. The requirement for actin in the recruitment of PIN s to the plasma membrane has been shown.11