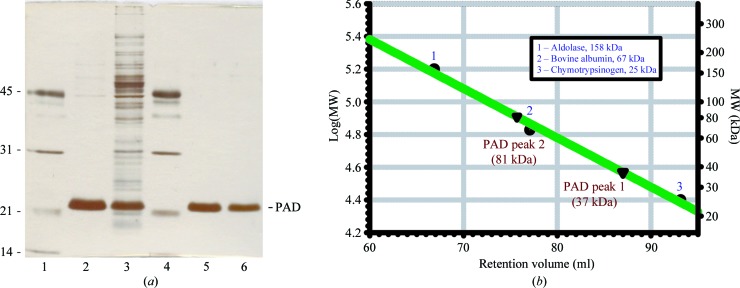
Figure 1.
Purification of recombinant B. pumilus PAD expressed in E. coli. (a) SDS–PAGE of PAD. Lanes 1 and 4, molecular-mass standards as indicated on the left (kDa); lane 3, crude extract; lane 2, after DEAE-Sepharose chromatography; lane 5, peak 1 after Butyl-S chromatography; lane 6, peak 2 after Butyl-S chromatography. (b) Gel-filtration chromatography on Sephadex 200 pg. The two active enzyme fractions that show a single band on SDS–PAGE (lanes 5 and 6) differ in their native molecular weight (37 and 81 kDa, respectively). Molecular-mass markers are indicated as follows: (1) aldolase (158 kDa), (2) bovine albumin (67 kDa), (3) chymotrypsin (25 kDa).