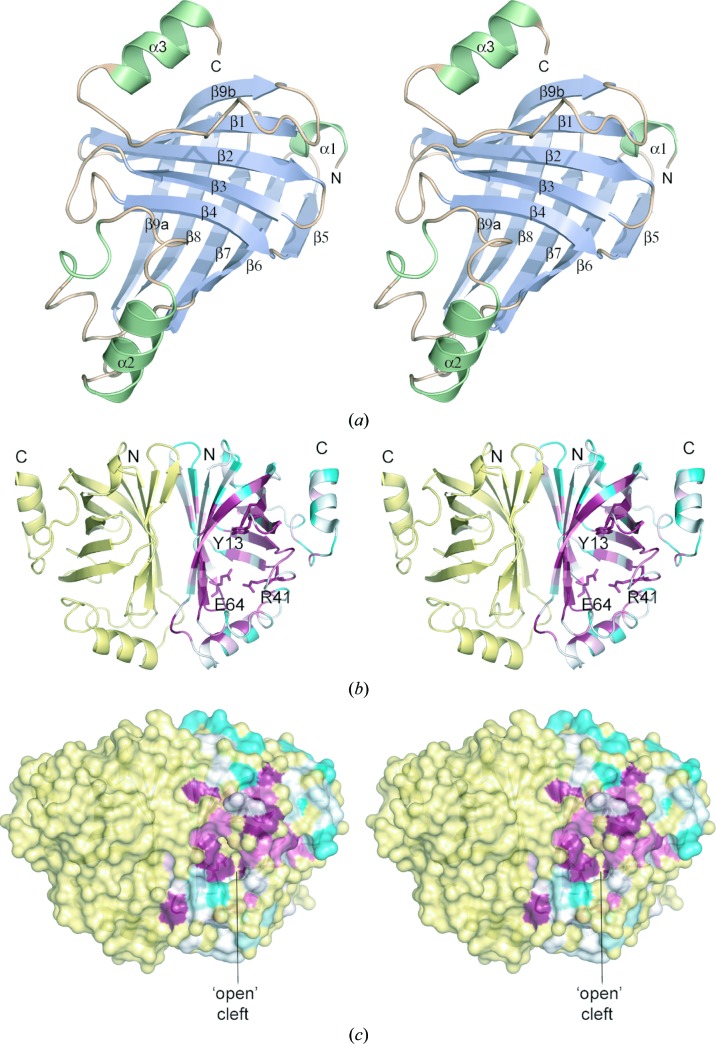
Figure 2.
Crystal structure of B. pumilus PAD. (a) Structure of the PAD monomer with secondary-structure elements coloured pale blue (β-strands) or light green (α-helices). (b) Structure of the PAD dimer, with subunit A coloured by sequence conservation using the ConSurf server (http://consurf.tau.ac.il/; white represents unconserved and dark magenta represents highly conserved). The dimer is oriented to show the twofold axis relating the two monomers. Key active-site residues are shown in stick representation. (c) Molecular-surface representation of PAD coloured as in (b) showing the ‘open’ conformation of the active-site cleft in subunit A. These and subsequent figures were prepared using the program PyMOL (http://www.pymol.org).