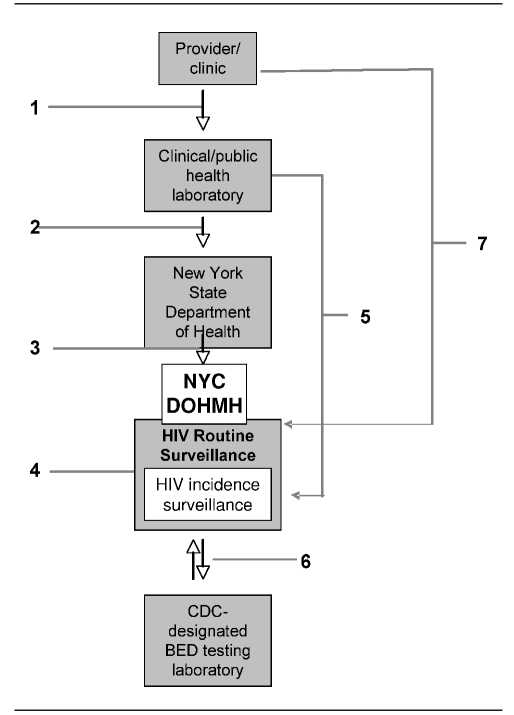
Figure 1.
Serologic testing algorithm for recent HIV seroconversion data flowa
a(1) Providers and clinics send blood samples to commercial clinical laboratories or the NYC DOHMH public health laboratory (PHL) for HIV diagnostic testing. (2) Commercial clinical laboratories or NYC DOHMH PHL report new diagnoses to the New York State Department of Health (NYSDOH). (3) NYSDOH reports new diagnoses to the NYC DOHMH HIV/AIDS routine surveillance (HRS). (4) HRS de-duplicates and verifies new diagnoses. (5) Commercial clinical laboratories or NYC DOHMH PHL ship remnant samples from new HIV diagnoses to the HIV Incidence Surveillance unit. (6) The Incidence Surveillance Unit ships eligible specimens to the Centers for Disease Control and Prevention-designated BED testing laboratory for BED testing, receives BED results, and calculates incidence. (7) Providers send testing and treatment history information for new diagnoses to HRS.
HIV = human immunodeficiency virus
NYC = New York City
DOHMH = Department of Health and Mental Hygiene
CDC = Centers for Disease Control and Prevention
BED = HIV subtype B, E, and D