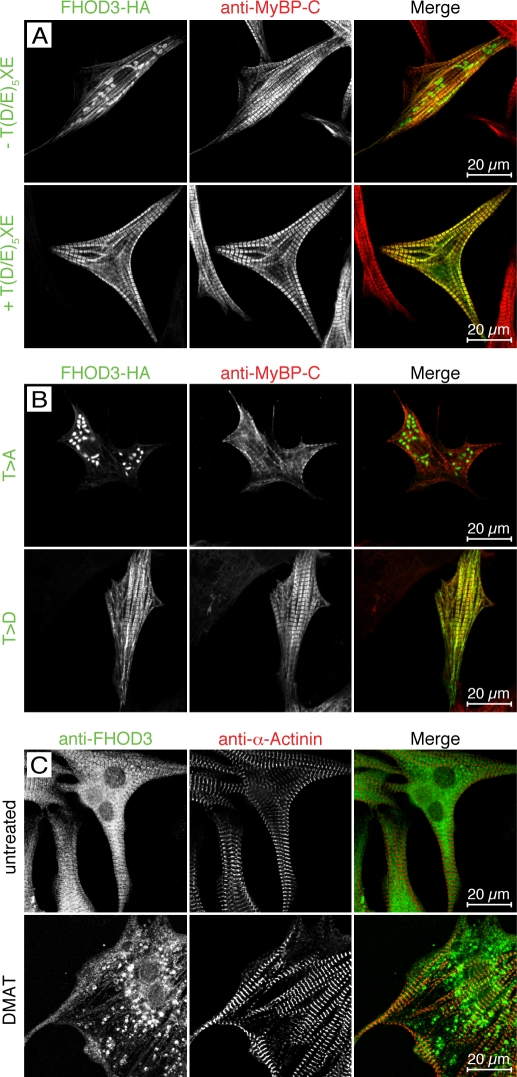
Figure 2.
The muscle-specific FHOD3 isoform is targeted to the sarcomere in a phosphorylation-dependent fashion. (A and B) NRCs were transfected with FHOD3-HA including or lacking the alternative T(D/E)5XE exon (A) or after mutation of residues T1474 and T1476 to alanine (T>A) or aspartate (T>D; B). After 48 h, cells were fixed and stained with monoclonal rat anti-HA and pRb anti–myosin-binding protein C (MyBP-C). Constructs including the T(D/E)5XE exon targeted to the A band, whereas constructs lacking the T(D/E)5XE exon formed cytoplasmic aggregates. Mutation of the threonine residues to alanine induced the formation of cytoplasmic aggregates as well. No change in localization was seen after mutation of the threonine residues to aspartic acid. (C) Untransfected NRCs were treated with 10 µM of the CK2 inhibitor DMAT for 24 h and then fixed and stained with anti-FHOD3 and anti–α-actinin antibodies. Untreated cells were used as a control. Similar to exogenous FHOD3, endogenous FHOD3 localizes to the A band but forms aggregates when phosphorylation by CK2 is inhibited.